ABSTRACT
Background
Currently, JAK-inhibitors are repurposed for therapy of Covid-19 because of their ability in restraining immune response, yet the corroboration regarding their advantage is still unclear. This study sought to analyze the efficacy of JAK-inhibitors to ameliorate the outcomes of Covid-19 sufferer.
Research design and methods: Using specific keywords, we comprehensively go through the potential articles on ClinicalTrials.gov, Europe PMC, and PubMed sources until June 2nd, 2021. All published studies on JAK-inhibitors and Covid-19 were collected.
Results
There were 14 studies with 4,363 Covid-19 patients contained in the meta-analysis. Based on our data, we suggested that JAK-inhibitors corresponded with increased recovery rate (RR 1.17; 95%CI: 1.01–1.36, p= 0.040, I2 = 91%, random-effect modeling); shortened time to recovery (mean difference −0.96; 95%CI: −1.15, −0.77, p< 0.00001, I2 = 28%, random-effect modeling); reduction of clinical deterioration risk (RR 0.66; 95%CI: 0.48–0.89, p= 0.008, I2 = 57%, random-effect modeling); and reduction of Covid-19 mortality (RR 0.52; 95%CI: 0.36–0.76, p= 0.0006, I2 = 33%, random-effect modeling).
Conclusions
This study propose that JAK-inhibitors perhaps provide advantageous effects on Covid-19 outcomes. JAK-inhibitors may be given during 1–2 weeks of disease to optimize its beneficial effects in halting the exaggerated immune response.
KEYWORDS: Coronavirus disease 2019, Covid-19, JAK-inhibitors, baricitinib, treatment
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection continues to spread globally causing a global pandemic and has become a major medical attention for the last couple years. The pandemic has put together over 169 million confirmed cases, with more than 3.5 million demise as of June 1st, 2021 [1]. While some severe acute respiratory syndrome coronavirus 2 infections appear as mild upper respiratory symptoms and may be self-limiting, there are still notable number of patients require hospitalizations and intensive treatment following progression into a more severe cases, varying from simple lower respiratory tract infections to acute respiratory distress syndrome (ARDS) and eventually may turn into multi-organ failure (MOF) [2,3].
Individuals with comorbidities such as chronic respiratory disease, diabetes, cardiovascular disease, obesity, and other immunocompromising conditions are facing higher risks in developing the severe form of SARS-CoV-2 infections [4–8]. The major factor responsible for the development of severe SARS-CoV-2 infections is the so-called ‘cytokine storm,’ which is characterized by exaggerated increase in the pro-inflammatory cytokines/chemokines from the abnormal host immune response [9,10]. For the past months, medical therapies to treat Covid-19 have been growing and evolving rapidly, ranging from supportive care, antiviruses, and anti-inflammatory agents [11–14]. Janus kinases (JAKs) are intracellular proteins that transduce and amplify signals from cytokines and growth factors. Their inhibitors, named Januspassw kinase (JAK)-inhibitors, have shown significant benefit and have been used in treating several autoimmune diseases and inflammatory diseases, like rheumatoid arthritis, inflammatory bowel diseases, and ankylosing spondylitis [15–17]. Recently, JAK-inhibitors have been repurposed for the management of Covid-19 because their ability to restrains immune response and cytokine release syndrome (CRS) during the route of Covid-19 [18]. These properties may offer clinical benefit in preventing the deterioration of coronavirus disease 2019 (Covid-19) and reducing the death toll from Covid-19 [18]. Moreover, when compared with other potential drugs for Covid-19, JAK-inhibitors have key pharmacological features for a potentially successful repurposing: convenient oral administration, favorable pharmacokinetic profile, and multifunctional pharmacodynamics by exerting dual anti-inflammatory and anti-viral effects [19]. However, the evidence regarding the advantage of JAK-inhibitors, particularly in Covid-19 patients, remains obscure. The purpose of this systematic review and meta-analysis is to come up with the evidence whether administration of JAK-inhibitors could enhance the outcomes of Covid-19 derived from the obtainable research.
2. Materials and methods
2.1. Eligibility criteria
This is a systematic review and meta-analysis study of the observational and clinical trial studies. We registered this study protocol in PROSPERO (CRD42021258750). Append research in this systematic review and meta-analysis were chose as most likely attaining the coming criteria: follow the PICO framework (P: Populations – hospitalized coronavirus disease 2019 patients; I: Interventions – treatments with JAK-inhibitors drugs (baricitinib, ruxolitinib, tofacitinib, or fedratinib); C: Comparator/Control – a group of patients who only receive standard of care therapy or any other medications as control/placebo and did not receive JAK-inhibitor drugs; O: Outcomes – recovery rate, time to recovery, clinical deterioration, and mortality), cross-sectional, case-control, cohort, and randomized or non-randomized clinical trial researches were contained. All studies besides original articles (correspondence or review articles), case-series or case report studies, studies reported other than in English language, research focusing on the pregnant women and populations of age below 18 years old were excluded.
2.2. Search strategy and study selection
The research from three databases (ClinicalTrials.gov, Europe PMC, and PubMed) was searched systemically. We used keywords ‘JAK-inhibitor’ OR ‘Janus Kinase Inhibitor’ OR ‘baricitinib’ OR ‘ruxolitinib’ OR ‘tofacitinib’ OR ‘fedratinib’ AND ‘SARS-CoV-2,’ OR ‘coronavirus disease 2019’ OR ‘Covid-19’ in a period from 2019 until June 2nd, 2021 with English-language restriction. Our searching strategy feature is listed in Table 1. Initial screening of titles and abstracts was conducted to identify eligible articles. Searches of potential articles were also done by analyzing the list of references of eligible studies. The search strategy we used was shown in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram.
Table 1.
Literature search strategy
| Database | Keyword | Result |
|---|---|---|
| PubMed | (‘janus kinase inhibitors’[All Fields] OR ‘janus kinase inhibitors’[MeSH Terms] OR (‘janus’[All Fields] AND ‘kinase’[All Fields] AND ‘inhibitors’[All Fields]) OR ‘janus kinase inhibitors’[All Fields] OR (‘jak’[All Fields] AND ‘inhibitor’[All Fields]) OR ‘jak inhibitor’[All Fields]) OR (‘baricitinib’[Supplementary Concept] OR ‘baricitinib’[All Fields]) OR (‘INCB018424’[Supplementary Concept] OR ‘INCB018424’[All Fields] OR ‘ruxolitinib’[All Fields]) OR (‘tofacitinib’[Supplementary Concept] OR ‘tofacitinib’[All Fields]) OR (‘Fedratinib’[Supplementary Concept] OR ‘Fedratinib’[All Fields] OR ‘fedratinib’[All Fields]) AND (‘COVID-19’[All Fields] OR ‘COVID-19’[MeSH Terms] OR ‘COVID-19 Vaccines’[All Fields] OR ‘COVID-19 Vaccines’[MeSH Terms] OR ‘COVID-19 serotherapy’[All Fields] OR ‘COVID-19 Nucleic Acid Testing’[All Fields] OR ‘covid-19 nucleic acid testing’[MeSH Terms] OR ‘COVID-19 Serological Testing’[All Fields] OR ‘covid-19 serological testing’[MeSH Terms] OR ‘COVID-19 Testing’[All Fields] OR ‘covid-19 testing’[MeSH Terms] OR ‘SARS-CoV-2’[All Fields] OR ‘sars-cov-2’[MeSH Terms] OR ‘Severe Acute Respiratory Syndrome Coronavirus 2’[All Fields] OR ‘NCOV’[All Fields] OR ‘2019 NCOV’[All Fields] OR ((‘coronavirus’[MeSH Terms] OR ‘coronavirus’[All Fields] OR ‘COV’[All Fields]) AND 2019/11/01[PubDate]: 3000/12/31[PubDate])) | 303 |
| Europe PMC | ‘JAK-inhibitor’ OR ‘Janus Kinase Inhibitor’ OR ‘baricitinib’ OR ‘ruxolitinib’ OR ‘tofacitinib’ OR ‘fedratinib’ AND ‘SARS-CoV-2,’ OR ‘coronavirus disease 2019’ OR ‘Covid-19’ | 1666 |
| ClinicalTrials.gov | ‘JAK-inhibitor’ OR ‘Janus Kinase Inhibitor’ OR ‘baricitinib’ OR ‘ruxolitinib’ OR ‘tofacitinib’ OR ‘fedratinib’ AND ‘SARS-CoV-2,’ OR ‘coronavirus disease 2019’ OR ‘Covid-19’ | 46 |
2.3. Data extraction and quality assessment
Two authors conducted the data extraction. We developed an extraction form to list the information about the study and its population characteristic, type of JAK-inhibitors drugs, JAK-inhibitors dose, the number of patients receiving JAK inhibitor, and the control group, as well as the outcome of coronavirus disease 2019 patients.
We are focus the outcomes on the recovery rate, time to recovery, clinical deterioration, and the mortality. The recovery rate is defined by the number of patients who achieve clinical improvements in terms of oxygenation and respiratory supports needed or the number of patients who were subsequently discharged from the hospitals during the follow-up period. Time to recovery is defined by the time patients needed to achieve the recovery state as defined above. Clinical deterioration outcome is defined by the clinical progression into a more severe disease state of Covid-19 as stated in to the Guidelines for the Diagnosis and Treatment of New Coronavirus Pneumonia (fifth edition) [20], admission to intensive care unit (ICU), and/or new use of mechanical ventilation. The mortality outcome described by the amount of patients who died during the follow-up period with positive coronavirus disease 2019.
Two authors assessed the quality of each study involved in this review independently. Modified Jadad scale was used to evaluate the quality of clinical trials including the allocation concealment, blindness, random allocation, withdrawals, and drop-outs of each study. The studies were scored starting from zero until seven and a research ranked as a high-quality research if the score were >4 [21]. Newcastle–Ottawa Scale (NOS) was used to evaluate the quality of case-control and cohort studies. The assessment reviews the comparability, selection, and outcome of each research, then each research was assigned a total score beginning with zero until nine. A research is graded as good quality if it scores ≥7 [22].
2.4. Statistical analysis
We used the Review Manager 5.4 (Cochrane Collaboration) software to perform the meta-analysis. To calculate risk ratio (RR) and its 95% confidence interval (95% CI) for the recovery rate, clinical deterioration, and mortality outcome we utilize Mantel-Haenszel’s formula. To obtain the mean difference (MD) and its standard deviations (SDs) for the time to recovery outcome, we used the Inverse Variance method. The I2 statistic was exerted to assess the heterogeneity with a value of <25% considered as low degree of heterogeneity, 26–50% moderate degree of heterogeneity, and >50% considered high degree of heterogeneity. Funnel plot analysis was utilized to assess the qualitative risk of publication bias, while the Begg and Mazumdar rank correlation test was used to assess the quantitative risk of publication bias [23].
3. Results
3.1. Study selection and characteristics
The searches on the databases yielded 2,015 studies. An overall of 1,632 study were attained after duplicates removing. As much as 1,567 records were removed after screening the titles and abstracts as well as matching the inclusion and exclusion criteria. Between 65 studies were full-text articles which were assessed for their eligibility, and 37 articles were excluded due to discouraging results (still recruiting or withdrawn subjects), seven articles had no control or comparison group, five articles did not bring up the criteria of the outcome of interest, two articles were not in English. This meta-analysis involved 14 studies [24–37] which included a total of 4,363 coronavirus disease 2019 patients (Figure 1). Out of 14 research, three research were double-blind randomized clinical trials, one research was a single-blind randomized clinical trial, two research were non-randomized clinical trials, five were prospective cohort research, and three were retrospective cohort research. Table 2 give out the characteristics of the included research.
Figure 1.
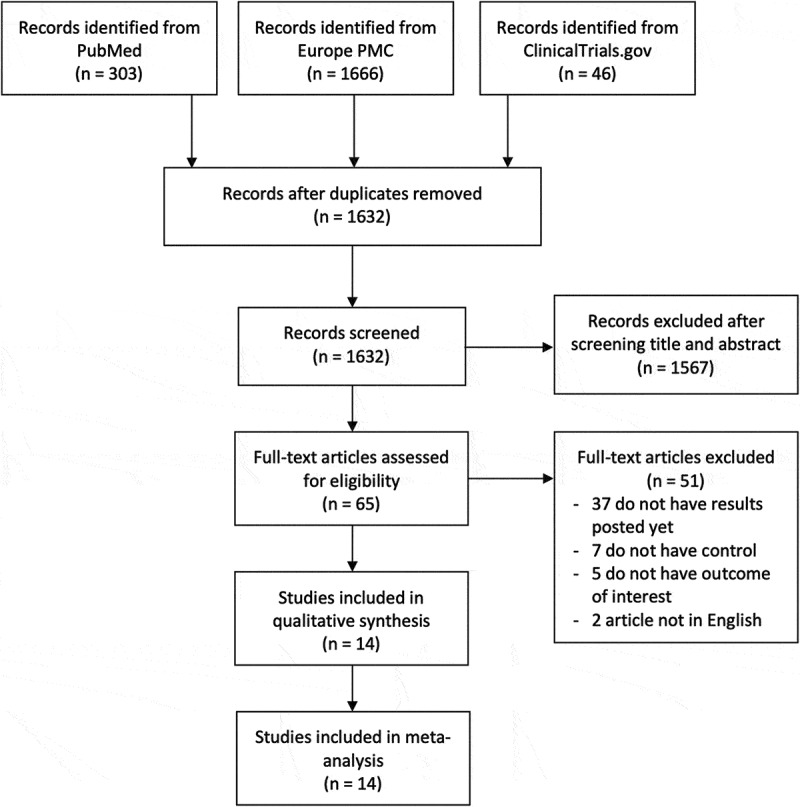
PRISMA diagram of the detailed process of selection of studies for inclusion in the systematic review and meta-analysis
Table 2.
Characteristics of included studies
| Study | Sample size | Design | Overall age mean ± SD | Outcome | Type of JAK-inhibitor | JAK-inhibitor dose | Patient category | Control | JAK-inhibitor vs control n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Bronte V et al[24]. 2020 | 76 | Prospective cohort | 73.5 ± 13.8 |
|
Baricitinib | 4 mg, twice daily for 2 days, followed by 4 mg/day for the remaining 7 days | Severe | Hydroxychloroquine ± lopinavir/ritonavir + supportive care | 20 (26.3%) vs 56 (73.7%) |
| Cantini F et al[25]. 2020 (a) | 24 | Non-randomized clinical trial | 64.4 ± 10.7 |
|
Baricitinib | 4 mg/day for 2 weeks | Mild tomoderate | Hydroxychloroquine + lopinavir/ritonavir | 12 (50%) vs 12 (50%) |
| Cantini F et al[26]. 2020 (b) | 191 | Retrospective cohort | 67 ± 14 |
|
Baricitinib | 4 mg/day for 2 weeks | Mild to Moderate | Hydroxychloroquine + lopinavir/ritonavir | 113 (59.1%) vs 78 (40.9%) |
| Cao Y et al[27]. 2020 | 41 | Single-blind Randomized clinical trial | 63 ± 7.4 |
|
Ruxolitinib | 5 mg, twice daily for 2 weeks | Severe | Vitamin C 100 mg as identical placebo + standard of care treatment | 20 (48.8%) vs 21 (51.2%) |
| D’Alessio A et al[28]. 2021 | 75 | Non-randomized clinical trial | 67.6 ± 5.1 |
|
Ruxolitinib | 5 mg, twice daily for 7 days, then tapered to 5 mg/day for a total of 10 days | Severe | Hydroxychloroquine ± lopinavir/ritonavir | 32 (42.6%) vs 43 (57.4%) |
| Giudice V et al[29]. 2020 | 17 | Prospective cohort | 63.5 ± 12.5 |
|
Ruxolitinib | 10 mg, twice daily for 14 days | Severe | Hydroxychloroquine + supportive care | 7 (41.1%) vs 10 (58.9%) |
| Kalil AC et al[30]. 2021 | 1033 | Double-blind randomized clinical trial | 55.4 ± 15.7 |
|
Baricitinib | 4 mg/day for 2 weeks | Moderate to severe | Identical placebo tablets | 515 (49.8%) vs 518 (50.2%) |
| Marconi VC et al[31]. 2021 | 1525 | Double-blind Randomized clinical trial | 57.6 ± 14.1 |
|
Baricitinib | 4 mg/day for 2 weeks | Moderate to severe | Identical placebo + standard of care treatment | 764 (50.1%) vs 761 (49.9%) |
| Maslennikov R et al[32]. 2021 | 62 | Retrospective cohort | 64.3 ± 12.5 |
|
Tofacitinib | 10 mg, twice daily on the first day, then 5 mg, twice daily for 4 days | Moderate to severe | Standard of care treatment | 32 (51.6%) vs 30 (48.4%) |
| Novartis Pharmaceuticals[33] 2021 | 432 | Double-blind randomized clinical trial | 56.5 ± 13.3 |
|
Ruxolitinib | 5 mg, twice daily for 2 weeks | Severe | Identical placebo + standard of care treatment | 287 (66.4%) vs 145 (33.6%) |
| Rodriguez-Garcia JL et al[34]. 2021 | 387 | Prospective cohort | 62.3 ± 14.8 |
|
Baricitinib | 4 mg/day for 5–10 days | Moderate to severe | Standard of care treatment + corticosteroids | 117 (30.2%) vs 270 (69.8%) |
| Rosas J et al[35]. 2020 | 29 | Retrospective cohort | 67.8 ± 13.6 | - Mortality | Baricitinib | 4 mg/day for 2 weeks | Moderate to severe | Standard of care treatment | 12 (41.3%) vs 17 (58.7%) |
| Stanevich OV et al[36]. 2021 | 292 | Prospective cohort | 58.1 ± 13.3 | - Mortality | Ruxolitinib | 5–10 mg/day until oxygen support withdrawal | Severe | Dexamethasone 16–24 mg/day for 5–10 days | 146 (50%) vs 146 (50%) |
| Stebbing J et al[37]. 2021 | 179 | Prospective cohort | 66 ± 26.6 |
|
Baricitinib | 4 mg/day for 2 weeks | Moderate to severe | Standard of care treatment | 37 (20.6%) vs 142 (79.4%) |
3.2. Quality of study assessment
We used Jadad scale assessment and suggested that four out of six clinical trial research were high quality, and the remaining two clinical trials were of low quality (Table 3). NOS scale was used to evaluate quality assessment of cohort and case-control studies, which indicated all included studies had good quality (Table 4).
Table 3.
Quality appraisal of studies included in the meta-analysis using Jadad scale asssessment
| Study | Random allocation |
Concealment schemes |
Blinding | Withdrawals and Drop-out | Total score | Interpretation |
|---|---|---|---|---|---|---|
| Cantini F et al.[25] 2020 (a) | 0 | 0 | 0 | 1 | 1 | Low quality |
| Cao Y et al.[27]2020 | 2 | 2 | 2 | 1 | 7 | High quality |
| D’Alessio A et al.[28]. 2021 | 0 | 0 | 0 | 1 | 1 | Low quality |
| Kalil AC et al.[30] 2021 | 1 | 1 | 2 | 1 | 5 | High quality |
| Marconi VC et al.[31] 2021 | 2 | 1 | 2 | 1 | 6 | High quality |
| Novartis Pharmaceuticals[33] 2021 | 1 | 1 | 2 | 1 | 5 | High quality |
Points were determined as follows: (1) random allocation: computer-generated random numbers, 2 points; not described, 1 point; inappropriate method, 0 point; (2) allocation concealment: central randomization, sealed envelopes or similar, 2 points; not described, 1 point; inappropriate or unused, 0 point; (3) blindness: identical placebo tablets or similar, 2 point; inadequate or not described, 1 point; inappropriate or no double blinding, 0 point; and (4) withdrawals and drop-outs: numbers and reasons are described, 1 point; not described, 0 point.
The Jadad scale score ranges from 1 to 7; higher score indicates better RCT quality. If a study had a modified Jadad score >4 points, it was considered to be of high quality; if the score was 3–4 points, it was moderate quality; and if the score was <3 points, it was low quality.
Table 4.
Newcastle-Ottawa quality assessment of observational studies
| First author, year | Study design | Selection | Comparability | Outcome | Total score | Result |
|---|---|---|---|---|---|---|
| Bronte V et al[24]. 2020 | Cohort | *** | ** | *** | 8 | Good |
| Cantini F et al[26]. 2020 (b) | Cohort | *** | ** | ** | 7 | Good |
| Giudice V et al[29]. 2020 | Cohort | *** | ** | ** | 7 | Good |
| Maslennikov R et al[32]. 2021 | Cohort | *** | ** | *** | 8 | Good |
| Rodriguez-Garcia JL et al[34]. 2021 | Cohort | *** | ** | *** | 8 | Good |
| Rosas J et al[35]. 2020 | Cohort | *** | ** | *** | 8 | Good |
| Stanevich OV et al[36]. 2021 | Cohort | *** | ** | ** | 7 | Good |
| Stebbing J et al[37]. 2021 | Cohort | *** | ** | *** | 8 | Good |
3.3. JAK-inhibitor and recovery rate
Seven studies (n = 3,320) reported recovery rate as the outcome of JAK- inhibitors in coronavirus disease 2019 patients. Our pooled analysis revealed that the treatment of JAK- inhibitors correlated with an enhanced recovery rate (RR 1.17; 95%CI: 1.01–1.36, p= 0.040, I2 = 91%, random-effect modeling) (Figure 2(a)).
Figure 2.
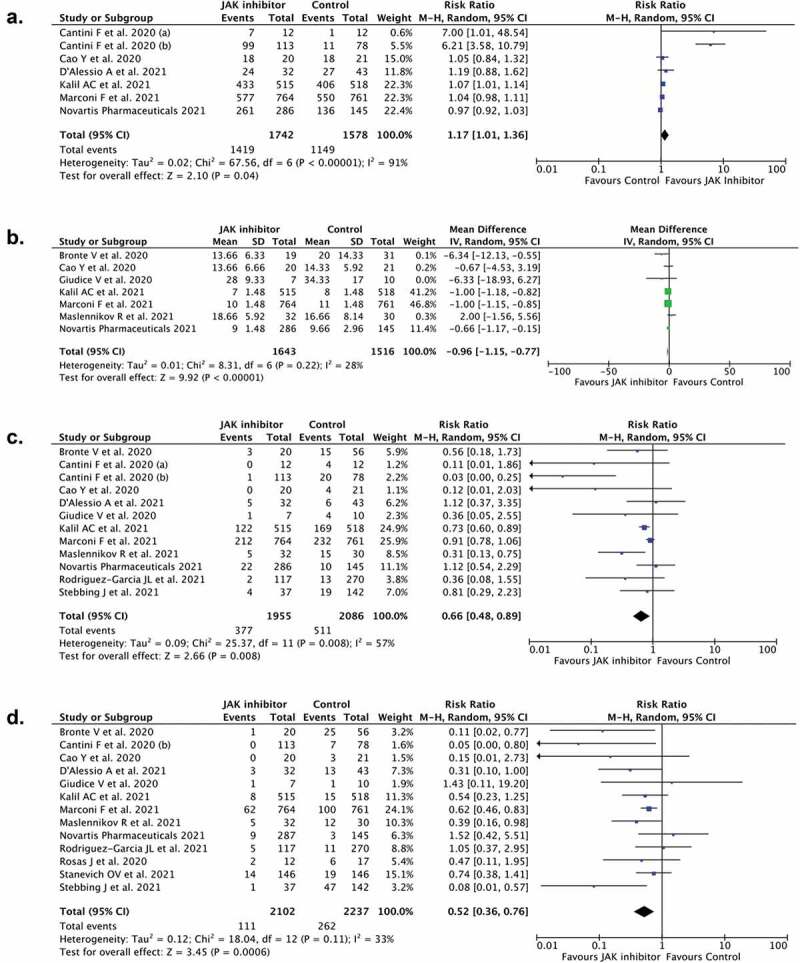
Forest plot that demonstrates the association of JAK-inhibitors administration with recovery rate (a), time to recovery (b), clinical deterioration (c), and mortality (d) outcomes
3.4. JAK-inhibitor and time to recovery
Seven studies (n = 3,159) reported the effect of JAK-inhibitors on the outcome of the time to recovery. The pooled analysis suggested that JAK-inhibitors treatment was correlated with shorter time to recovery (mean difference −0.96; 95%CI: −1.15, −0.77, p< 0.00001, I2 = 28%, random-effect modeling) (Figure 2(b)).
3.5. JAK-inhibitor and clinical deterioration of Covid-19
Twelve studies (n = 4,041) reported clinical deterioration as the outcome of JAK-inhibitor in Covid-19. The pooled estimate indicated that JAK-inhibitors treatment was correlated with reduction in risk of clinical deterioration in coronavirus disease 2019 (RR 0.66; 95%CI: 0.48–0.89, p= 0.008, I2 = 57%, random-effect modeling) (Figure 2(c)).
3.6. JAK-inhibitor and mortality of Covid-19 patients
The mortality outcome was revealed in thirteen studies (n = 4,339). The pooled estimate indicated that JAK-inhibitor treatment was associated with lower mortality from coronavirus disease 2019 (RR 0.52; 95%CI: 0.36–0.76, p= 0.0006, I2 = 33%, random-effect modeling) (Figure 2(d)).
3.7. Publication bias
We used Funnel plot analysis for the recovery rate (Figure 3(a)), time to recovery (Figure 3(b)), and mortality outcomes (Figure 3(d)). These analysis showed a relatively symmetrical inverted-plot, indicating no publication bias. Funnel plot analysis appeared an asymmetrical inverted-plot for the clinical deterioration outcome (Figure 3(c)), indicating some of publication bias. The result from Begg and Mazumdar rank-correlation test were not statistically significant for recovery rate (p = 0.133), time to recovery (p = 0.763), and mortality outcome (p = 0.099), confirming the results from funnel plot analysis in which no sign of publication bias was found. However, Begg and Mazumdar rank-correlation test were statistically significant for clinical deterioration outcome (p = 0.046), confirming the presence of publication bias.
Figure 3.
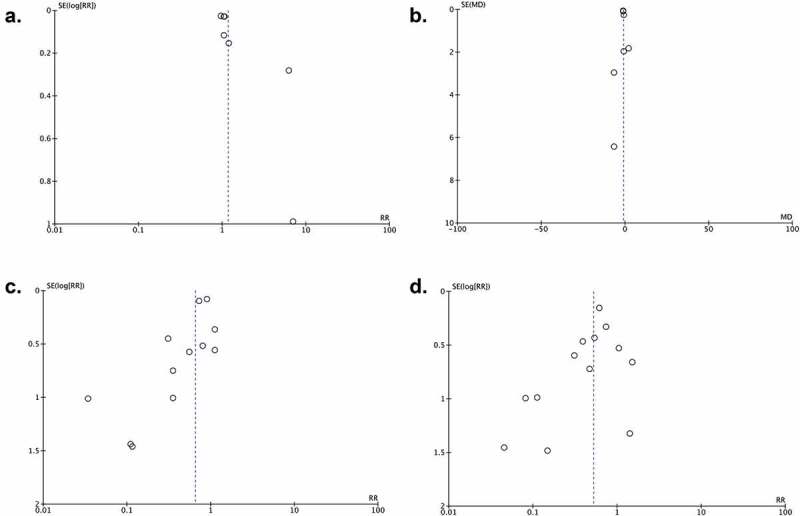
Funnel plot analysis for the association of JAK-inhibitors administration with recovery rate (a), time to recovery (b), clinical deterioration (c), and mortality (d) outcomes
4. Discussion
According to our pooled analysis, it was discovered that administration of JAK-inhibitors was associated with an enhanced recovery rate, shortened time to recovery, reduction risk of clinical deterioration, and lower mortality from coronavirus disease 2019.
There are some explanations how JAK-inhibitor could affect the prognosis of Covid-19 patients. Receptor-mediated endocytosis is the most common means of virus entrance into host cells. Severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) – the responsible pathogen for Covid-19 – used the surface receptor angiotensin-converting enzyme 2 (ACE2) to infect cells, especially AT2 alveolar epithelial cells in lungs [38]. AP2-associated protein kinase 1 (AAK1) is one of the known regulators of endocytosis. Interference of AAK1 might, in turn, cut in the movement of the virus into cells and also the intracellular congregation of virus particles [39]. Fourty-seven from a total of 378 AAK1 inhibitors have been accepted for medical use in which 6 of them can inhibit AAK1 with high affinity [18]. Baricitinib is one of the six high-affinity AAK1-binding drugs which also binds the regulator of endocytosis, cyclin G-associated kinase and therefore inhibits the SARS-CoV-2 endocytosis and inflammatory response caused by interaction between the virus and host immune systems [18]. Moreover, several studies have demonstrated that severe and deceased Covid-19 patients were characterized by elevations of inflammatory mediators like lactate dehydrogenase (LDH), C-reactive protein (CRP), procalcitonin, D-dimer, and the levels of several cytokines, like interleukin-2 (IL-2), IL-6, IL-10, granulocyte colony-stimulating factor (G-CSF), a tumor necrosis factor-α (TNF-α). This phenomenon is known as cytokine release syndrome (CRS) [40,41]. Hypercytokinemia or CRS was thought to be the underlying cause for clinical deterioration, development of complications like acute respiratory distress syndrome (ARDS), and responsible for the increased death tendency in coronavirus disease 2019 patients [41,42]. Meta-analysis study has also demonstrated that elevated IL-6 levels was associated with poor outcomes in Covid-19 [43]. JAK-inhibitors target JAK1, JAK2, and JAK3, which further downregulate the JAK/STAT signaling pathway, leading to decrease cytokine amounts, thus decreasing CRS [44–46]. Not only can JAK-inhibitors stop the action of IL-6, it also blocks most likely pathogenic cytokines, including IL-2, interferon-γ (IFN-γ), granulocyte-macrophage colony stimulating factor (GM-CSF), and granulocyte-colony stimulating factor (G-CSF) [44–46]. Given these benefits of JAK-inhibitor in interrupting viral entry into cells and controlling immune response, it is not surprising that JAK-inhibitor may ameliorate the coronavirus disease 2019 outcomes.
Theoretically, patients with Covid-19 patients would benefit from anti-inflammatory drugs if it were given at the right time. Siddiqi and Mehra have proposed a clinical staging system which consist of three Covid-19 stages in terms of illness development [47]. The third stage was marked by severe extrapulmonary systemic hyperinflammation syndrome, ARDS, systemic inflammatory response syndrome (SIRS), and impending multi-organ failure. It is believed that JAK-inhibitors should be considered prior to multiorgan dysfunction [47]. Hence, it is reasonable to consider that JAK-inhibitors would give benefit if given within 1–2 weeks of disease [48].
There are some limitations in this study. Notable heterogeneities were identified on most of the outcomes of interests included in this study. This might be due to difference in the type of JAK-inhibitors and in the dosage of JAK-inhibitors given to the patients. We also included some pre-print studies in our analysis. However, we have made rigorous endeavor to make certain that only meaningful research and several pre-print research were included to reduce publication bias risk. Finally, the results from our analysis were largely based on the observational studies because at this time only 6 clinical trials were available and have results posted. Further randomized clinical trial research are still required to validate the outcome from this analysis.
5. Conclusions
Our systematic review and meta-analysis indicated that the treatment of JAK-inhibitors showed favorable outcomes in Covid-19, in terms of recovery rate, shortened time to recovery, reduction risk of clinical deterioration, and reduction of mortality rate. These beneficial properties of JAK-inhibitors in Covid-19 course are closely related to their abilities in halting the exaggerated inflammatory response and cytokine release syndrome (CRS). Therefore, we propose that JAK-inhibitors should also be given in the right time to optimize its beneficial properties, preferably within 1–2 weeks of the disease, before the development of respiratory distress and multiple organ failure. This review also propose that JAK-inhibitors might be a prospect agent for the treatment of Covid-19. Even so, more randomized clinical trial studies are still required to further verify its benefit regarding to the timing of initiating treatment. Finally, JAK-inhibitors should be considered as a promising drug to improve the treatment as well as to decrease the mortality in Covid-19 patients.
Funding Statement
This paper was not funded.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Author contribution statement
All of the authors of this manuscript have (1) substantially contributed to the conception and design of the review article and interpreting the relevant literature and (2) been involved in writing the review article or revised it for intellectual content.
References
- 1.World Health Organization . Coronavirus disease (COVID-19): situation report. Accessed 2021 Jun 6. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—1-june-2021
- 2.Hariyanto TI, Rizki NA, Kurniawan A.. Anosmia/hyposmia is a good predictor of coronavirus disease 2019 (COVID-19) infection: a meta-analysis. Int Arch Otorhinolaryngol. 2020. DOI: 10.1055/s-0040-1719120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwenandar F, Japar KV, Damay V, et al. Coronavirus disease 2019 and cardiovascular system: a narrative review. Int J Cardiol Heart Vasc. 2020;29:100557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Putri C, Hariyanto TI, Hananto JE, et al. Parkinson’s disease may worsen outcomes from coronavirus disease 2019 (COVID-19) pneumonia in hospitalized patients: a systematic review, meta-analysis, and meta-regression. Parkinsonism Relat Disord. 2021. Apr 24;S1353-8020(21)00152–8. DOI: 10.1016/j.parkreldis.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hariyanto TI, Rosalind J, Christian K, et al. Human immunodeficiency virus and mortality from coronavirus disease 2019: a systematic review and meta-analysis. South Afr J HIV Med. 2021. Apr 15;22(1):1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hariyanto TI, Intan D, Hananto JE, et al. Pre-admission glucagon-like peptide-1 receptor agonist (GLP-1RA) and mortality from coronavirus disease 2019 (Covid-19): a systematic review, meta-analysis, and meta-regression. Diabetes Res Clin Pract. 2021;179:109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gold MS, Sehayek D, Gabrielli S, et al. COVID-19 and comorbidities: a systematic review and meta-analysis. Postgrad Med. 2020. Nov;132(8):749–755. [DOI] [PubMed] [Google Scholar]
- 8.Hariyanto TI, Kurniawan A. Obstructive sleep apnea (OSA) and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: a systematic review and meta-analysis. Sleep Med. 2021Jun;82:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castelli V, Cimini A, Ferri C. Cytokine storm in COVID-19: “when you come out of the storm, you won’t be the same person who walked in” Front Immunol. 2020. Sep 2;11: 2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang Y, Liu J, Zhang D, et al. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hariyanto TI, Hardyson W, Kurniawan A. Efficacy and safety of tocilizumab for coronavirus disease 2019 (Covid-19) patients: a systematic review and meta-analysis. Drug Res (Stuttg). 2020. DOI: 10.1055/a-1336-2371 [DOI] [PubMed] [Google Scholar]
- 12.Ivan Hariyanto T, Kurniawan A. Tocilizumab administration is associated with the reduction in biomarkers of coronavirus disease 2019 infection. J Med Virol. 2021. Mar;93(3):1832–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hariyanto TI, Halim DA, Jodhinata C, et al. Colchicine treatment can improve outcomes of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Clin Exp Pharmacol Physiol. 2021. Jun;48(6):823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hariyanto TI, Halim DA, Rosalind J, et al. Ivermectin and outcomes from Covid‐19 pneumonia: a systematic review and meta‐analysis of randomized clinical trial studies. Rev Med Virol. 2021. 6;Jun:e2265. [Google Scholar]
- 15.O’Shea JJ, Kontzias A, Yamaoka K, et al. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis. 2013. Apr;72(Suppl 2(0 2)):ii111–5. 10.1136/annrheumdis-2012-202576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunwar S, Collins CE, Constantinescu F. Baricitinib, a Janus kinase inhibitor, in the treatment of rheumatoid arthritis: a systematic literature review and meta-analysis of randomized controlled trials. Clin Rheumatol. 2018. Oct;37(10):2611–2620. [DOI] [PubMed] [Google Scholar]
- 17.Sands BE, Sandborn WJ, Feagan BG, et al. Peficitinib, an oral janus kinase inhibitor, in moderate-to-severe ulcerative colitis: results from a randomised, phase 2 study. J Crohns Colitis. 2018. Nov 9;12(10):1158–1169. [DOI] [PubMed] [Google Scholar]
- 18.Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020. Feb 15;395(10223):e30–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gatti M, Turrini E, Raschi E, et al. Janus kinase inhibitors and coronavirus disease (COVID)-19: rationale, clinical evidence and safety issues. Pharmaceuticals (Basel). 2021. Jul 28;14(8):738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Health Commission of the People’s Republic of China . Diagnosis and treatment of new coronavirus pneumonitis. (trial version 5). http://www.nhc.gov.cn/yzygj/s7653p/202002/3b09b894ac9b4204a79db5b8912d4440.shtml
- 21.Huang J, Wang X, Chen X, et al. Perioperative antibiotics to prevent acute endophthalmitis after ophthalmic surgery: a systematic review and meta-analysis. PLoS One. 2016. Nov 8;11(11):e0166141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Margulis AV, Pladevall M, Riera-Guardia N, et al. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle-Ottawa Scale and the RTI item bank. Clin Epidemiol. 2014;6:359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994. Dec;50(4):1088–1101. [PubMed] [Google Scholar]
- 24.Bronte V, Ugel S, Tinazzi E, et al. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J Clin Invest. 2020. Dec 1;130(12):6409–6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cantini F, Niccoli L, Matarrese D, et al. Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact. J Infect. 2020. Aug;81(2):318–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cantini F, Niccoli L, Nannini C, et al. Beneficial impact of baricitinib in COVID-19 moderate pneumonia; multicentre study. J Infect. 2020. Oct;81(4):647–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao Y, Wei J, Zou L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol. 2020. Jul;146(1):137–146.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Alessio A, Del Poggio P, Bracchi F, et al. Low-dose ruxolitinib plus steroid in severe SARS-CoV-2 pneumonia. Leukemia. 2021. Feb;35(2):635–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giudice V, Pagliano P, Vatrella A, et al. Combination of ruxolitinib and eculizumab for treatment of severe SARS-CoV-2-related acute respiratory distress syndrome: a controlled study. Front Pharmacol. 2020;11:857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus Remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021. Mar 4;384(9):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marconi VC, Ramanan AV, De Bono S, et al. Efficacy and safety of baricitinib in patients with COVID-19 infection: results from the randomised, double-blind, placebo-controlled, parallel-group COV-BARRIER phase 3 trial. medRxiv. 2021. 10.1101/2021.04.30.21255934 [DOI] [PMC free article] [PubMed]
- 32.Maslennikov R, Ivashkin V, Vasilieva E, et al. Tofacitinib reduces mortality in coronavirus disease 2019 Tofacitinib in COVID-19. Pulm Pharmacol Ther. 2021;69:102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novartis Pharmaceuticals . Phase 3 randomized, double-blind, placebo-controlled multi-center study to assess the efficacy and safety of ruxolitinib in patients with COVID-19 associated cytokine storm (RUXCOVID). ClinicalTrials.gov. NCT04362137. https://clinicaltrials.gov/ct2/show/results/NCT04362137?term=ruxolitinib&cond=Covid19&draw=2&rank=8
- 34.Rodriguez-Garcia JL, Sanchez-Nievas G, Arevalo-Serrano J, et al. Baricitinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: an observational cohort study. Rheumatology (Oxford). 2021. Jan 5;60(1):399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosas J, Liaño FP, Cantó ML, et al. Experience with the use of baricitinib and tocilizumab monotherapy or combined, in patients with interstitial pneumonia secondary to coronavirus COVID19: a real-world study. Reumatol Clin (Engl Ed). 2020. Nov 28;S1699-258X(20)30271–0. DOI: 10.1016/j.reuma.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stanevich OV, Fomina DS, Bakulin IG, et al. Ruxolitinib versus dexamethasone in hospitalized adults with Covid-19: multicenter matched-controlled study. medRxiv. 2021. 10.1101/2021.04.20.21255662 [DOI] [PMC free article] [PubMed]
- 37.Stebbing J, Sánchez Nievas G, Falcone M, et al. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. 2021. Jan 1;7(1):eabe4724. 10.1126/sciadv.abe4724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hariyanto TI, Japar KV, Damay V, et al. The use of ACE inhibitor/ARB in SARS-CoV-2 patients: a comprehensive narrative review. Asian J Med Sci. 2020;11(6):113–120. [Google Scholar]
- 39.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020. Feb 22;395(10224):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hariyanto TI, Japar KV, Kwenandar F, et al. Inflammatory and hematologic markers as predictors of severe outcomes in COVID-19 infection: a systematic review and meta-analysis. Am J Emerg Med. 2021;41:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hojyo S, Uchida M, Tanaka K, et al. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 2020;40:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Picchianti Diamanti A, Rosado MM, Pioli C, et al. Cytokine release syndrome in COVID-19 patients, a new scenario for an old concern: the fragile balance between infections and autoimmunity. Int J Mol Sci. 2020. May 8;21(9):3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coomes EA, Haghbayan H. Interleukin-6 in Covid-19: a systematic review and meta-analysis. Rev Med Virol. 2020. Nov;30(6):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peterson D, Damsky W, King B. The use of Janus kinase inhibitors in the time of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). J Am Acad Dermatol. 2020. Jun;82(6):e223–e226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Solimani F, Meier K, Ghoreschi K. Janus kinase signaling as risk factor and therapeutic target for severe SARS-CoV-2 infection. Eur J Immunol. 2021. May;51(5):1071–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peterson D, Damsky W, King B. Reply: calm before the storm: understanding the role of Janus kinase inhibitors in COVID-19. J Am Acad Dermatol. 2020. Jul;83(1):e67–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020. May;39(5):405–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang W, Zhao Y, Zhang F, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol. 2020May;214:108393. [DOI] [PMC free article] [PubMed] [Google Scholar]


