ABSTRACT
Clinically significant hepatic acquired immunodeficiency syndrome–related Kaposi sarcoma is rarely described in the literature. Kaposi sarcoma immune reconstitution inflammatory syndrome may play a role in the rapid progression of clinically insignificant to significant liver disease. We present an acquired immunodeficiency syndrome patient with disseminated Kaposi sarcoma that developed 3–6 weeks after initiation of highly active antiretroviral therapy.
INTRODUCTION
AIDS-related Kaposi sarcoma (KS) prevalence has decreased since the introduction of highly active antiretroviral therapy (HAART) but remains one of the most common tumors to develop in patients with acquired immunodeficiency syndrome (AIDS).1 KS risk factors include men who have sex with men, human immunodeficiency virus (HIV) patients on HAART with a CD4 count <200 cells/μL, HIV patients on steroid therapy, and a history of other sexually transmitted diseases.2,3 KS pathogenesis is multifactorial involving human herpes virus-8 (HHV-8) which mimics oncogenes and activates viral and host cytokines that promote KS cell proliferation.4 Cutaneous disease is the most common manifestation followed by visceral disease. Of potential visceral KS sites, the gastrointestinal tract is most frequently involved followed by the lungs.5 Up to 35% of KS patients have hepatic involvement diagnosed on incidental imaging or autopsy.2 Hepatic disease is rarely clinically significant, but if present, may progress to hepatic failure, multiorgan dysfunction, and death.2 Immune reconstitution inflammatory syndrome (IRIS), a syndrome preceded by HAART initiation in HIV-infected individuals with paradoxical worsening of pre-existing infection, has been seldom described to unmask or exacerbate KS.6 We describe a rare case of disseminated KS-IRIS with significant infiltrative and cholestatic liver disease.
CASE REPORT
A 48-year-old man with a past medical history of HIV, diagnosed 6 weeks before admission, presented to the hospital with a 3-week history of generalized abdominal pain, weight loss, and jaundice. He had no significant surgical or family history. His social history was remarkable for unprotected sexual intercourse with multiple male partners over the past 5 months before admission. Home medications included a combination of bictegravir, emtricitabine, and tenofovir and trimethoprim-sulfamethoxazole. His vital signs on arrival included blood pressure of 137/92 mm Hg, heart rate of 104, a body temperature of 98.5°F, respiratory rate of 20 breaths per minute, and SpO2 99% on room air. Physical examination was remarkable for scleral icterus, mild to moderate right upper quadrant abdominal pain and bilateral inguinal lymphadenopathy. No skin or oral lesions were noted.
On HIV diagnosis, the patient reported having fatigue and a 10-lb. weight loss, but no other gastrointestinal symptoms. His CD4 count and HIV viral load at diagnosis were 21 cells/μL and 554,635 copies/mL. On this admission, his CD4 count and HIV viral load were 121 cells/μL and 659 copies/mL. His white blood cell count was 7.68 K/μL, hemoglobin 7.4 g/dL, platelet count 38 K/μL, blood urea nitrogen 23 mg/dL, and creatinine 1.14 mg/dL. In addition, albumin was 2.2 g/dL, total bilirubin 14.8 mg/dL, direct bilirubin 10.19 mg/dL, alkaline phosphatase 324 U/L, aspartate aminotransferase 89 U/L, alanine aminotransferase 164 U/L, and international normalized ratio of 1.23. Notably, his liver function tests, before taking HAART therapy, were normal. Infectious workup including QuantiFERON TB gold, serum cryptococcal antigen, urine histoplasma antigen, hepatitis A, B, and C, cytomegalovirus and Epstein-Barr virus serologies, and peripheral and acid-fast bacilli blood cultures was negative.
Abdominal and pelvic computed tomography (CT) with intravenous contrast demonstrated a 1.2 × 1.2-cm hypodense lesion in the anterior-inferior left liver lobe with multiple other liver hypodensities (Figure 1). Magnetic resonance cholangiopancreatography showed mild to moderate bilateral intrahepatic biliary ductal dilation and patchy T1-hypointense liver lesions (Figure 2). Liver biopsy of the 1.2 × 1.2-cm lesion as seen on CT abdomen and pelvis and left Inguinal lymph node biopsy showed KS with positive HHV-8 and CD31 immunostaining (Figure 3).
Figure 1.
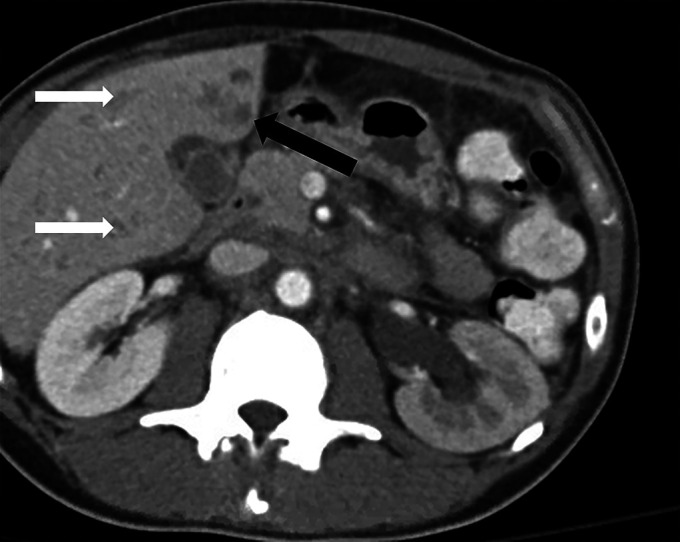
Axial view of the abdominal and pelvic computed tomography with intravenous contrast demonstrating a 1.2 × 1.2-cm hypodense lesion (black arrow) in the anterior-inferior left hepatic lobe. Additional scattered subcentimeter hypodensities (white arrows) are also seen in the liver.
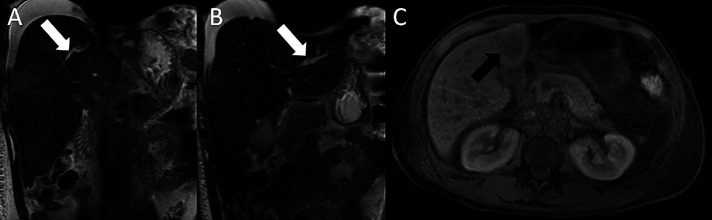
Figure 2:
(A) Magnetic resonance cholangiopancreatography (T2 coronal view) showing mild to moderately dilated right intrahepatic biliary duct (white arrow). (B) Magnetic resonance cholangiopancreatography (T2 coronal view) showing mild to moderately dilated left intrahepatic biliary duct (white arrow). (C) Magnetic resonance cholangiopancreatography (T1 axial view) showing an ill-defined hypointense lesion in the anterior-inferior hepatic lobe (black arrow).
Figure 3.
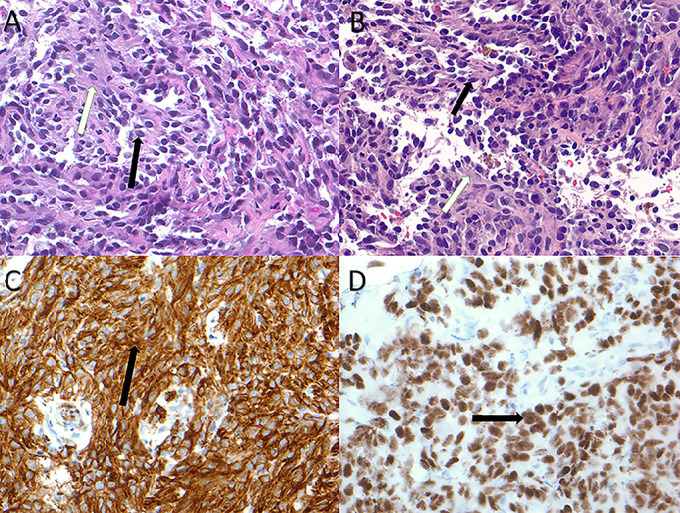
(A) Liver lesion biopsy demonstrating medium-sized ovoid endothelial cells of variable cellularity (black arrow) infiltrating fibroconnective tissue (white arrow) (hematoxylin and eosin stain). (B) L. inguinal lymph node biopsy demonstrating medium-sized ovoid endothelial cells of variable cellularity (black arrow) infiltrating fibroconnective tissue (white arrow) (hematoxylin and eosin stain). (C) Liver lesion biopsy demonstrating positive immunostaining for CD31 (black arrow) (CD31 immunostain). (D) Liver lesion biopsy demonstrating positive immunostaining for HHV-8 (black arrow) (HHV-8 immunostain). HHV-8, human herpes virus-8.
Esophagogastroduodenoscopy showed purple polypoid lesions in the duodenum with biopsy demonstrating KS and positive HHV-8 immunostaining (Figures 4 and 5). Endoscopic retrograde cholangiopancreatography was performed because of the biliary dilation and showed right and left intrahepatic duct stenosis; however, stents could not be placed because of the severity of stenosis. Given the concern for cholangitis, percutaneous transhepatic cholangiogram was performed, but biliary drain placement was aborted because of the procedure being complicated by hemoperitoneum. He clinically declined, and chemotherapy was not felt to be an appropriate option. After discussion with the patient and family, he was enrolled in hospice.
Figure 4.
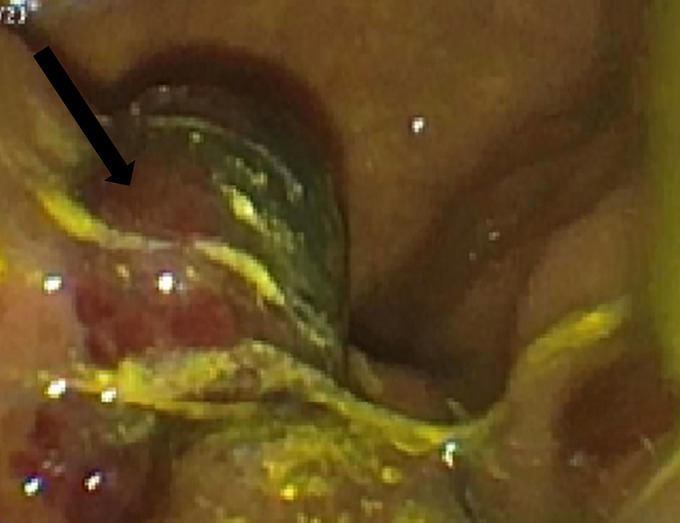
Esophagogastroduodenoscopy demonstrating a purplish, small- to medium-sized polypoid lesion (black arrow) in the duodenum.
Figure 5.
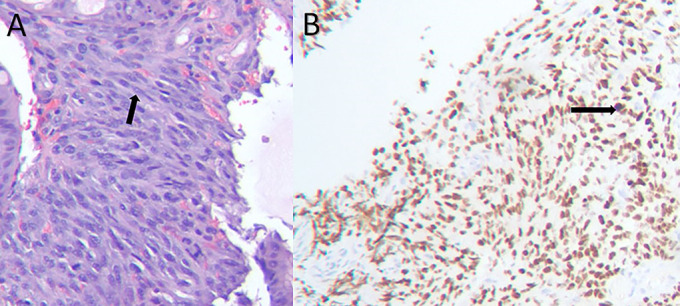
(A) Duodenal polypoid lesion biopsy (H + E stain) showing numerous spindle cells (black arrow). (B) Duodenal polypoid lesion biopsy (HHV-8 immunostain) showing positive immunostaining for HHV-8 (black arrow). HHV-8, human herpes virus-8.
DISCUSSION
Of the 4 variants, including “classical,” “African endemic,” “iatrogenic,” and “AIDS-related,” AIDS-related KS has the highest rate of hepatic involvement.2 AIDS-related hepatic KS is typically asymptomatic, thus rarely diagnosed. Incidence of hepatic KS is ascertained from small case series and reports. Prevalence is determined mostly from autopsy reports with up to 34% describing liver involvement.7–9 Hepatocyte growth factor, a kinase that mediates epithelial cell proliferation and angiogenesis, can induce HHV-8 lytic replication and provide a means for hepatic KS progression.10,11 In a review of 8 KS patients with clinically significant liver disease, 6 had cholestatic liver injury just as in our patient.2 Abdominal and liver CT findings include hepatomegaly with multiple small hypodense lesions often in the periportal area.12 Magnetic resonance imaging–specific liver findings include hypointense lesions on T1-weighted out-of-phase imaging.13 Liver biopsy demonstrates hyaline globules, hemosiderin accumulation, spindle cells with large, irregular nuclei, and positive immunostaining for HHV-8, CD31, and CD34.2 The AIDS clinical trial group of the National Institute of Health developed staging guidelines for dividing patients into prognostic categories.14 T0 KS is disease limited to skin or the oral cavity. T1 KS has visceral disease with more widespread involvement. I0 KS has a CD4 count >200, whereas I1 KS has a CD4 count <200. S0 KS has no B symptoms or history of opportunistic infection, whereas S1 KS patients have either.14 Our patient had T1I1S1 staging and thus a poor prognosis.
Hepatic KS treatment is no different than treatment for advanced KS.15 First-line therapy for advanced KS includes HAART therapy and pegylated liposomal doxorubicin, second line with paclitaxel, and third line including etoposide, bleomycin, vinblastine, and vincristine. Biologic and targeted molecular therapies have been studied with varying efficacy but can be considered if combination of HAART and chemotherapy fails.2 For KS-IRIS patients, some studies suggest HAART with chemotherapy can be effective, but >33% of patients may achieve remission with HAART alone.6,16,17 This case report highlights the importance of following liver function tests in HIV patients and KS-IRIS identification as there may be rapidly progressive disease. Further studies need to be completed regarding incidence, prevalence, mortality, and treatment of hepatic KS.
DISCLOSURES
Author contributions: J. Van reviewed the literature and wrote the manuscript. N. Reau edited the manuscript, revised the manuscript for intellectual content, approved the final manuscript, and is the author guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
REFERENCES
- 1.Stover KR, Molitorisz S, Swiatlo E, et al. A fatal case of Kaposi sarcoma due to immune reconstitution inflammatory syndrome. Am J Med Sci. 2012;343(5):421–5. [DOI] [PubMed] [Google Scholar]
- 2.Van Leer-Greenberg B, Kole A, Chawla S. Hepatic Kaposi sarcoma: A case report and review of the literature. World J Hepatol. 2017;9(4):171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trattner A, Hodak E, David M, et al. The appearance of Kaposi sarcoma during corticosteroid therapy. Cancer. 1993;72(5):1779–83. [DOI] [PubMed] [Google Scholar]
- 4.Gantt S, Casper C. Human herpesvirus 8-associated neoplasms: The roles of viral replication and antiviral treatment. Curr Opin Infect Dis. 2011;24(4):295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martellotta F, Berretta M, Vaccher E, et al. AIDS-related Kaposi's sarcoma: State of the art and therapeutic strategies. Curr HIV Res. 2009;7(6):634–8. [DOI] [PubMed] [Google Scholar]
- 6.Odongo FC. Fatal disseminated Kaposi's sarcoma due to immune reconstitution inflammatory syndrome following HAART initiation. Case Rep Infect Dis. 2013;2013:546578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niedt GW, Schinella RA. Acquired immunodeficiency syndrome. Clinicopathologic study of 56 autopsies. Arch Pathol Lab Med. 1985;109(8):727–34. [PubMed] [Google Scholar]
- 8.Lemlich G, Schwam L, Lebwohl M. Kaposi's sarcoma and acquired immunodeficiency syndrome. Postmortem findings in twenty-four cases. J Am Acad Dermatol. 1987;16(2):319–25. [DOI] [PubMed] [Google Scholar]
- 9.Schneiderman DJ, Arenson DM, Cello JP, et al. Hepatic disease in patients with the acquired immune deficiency syndrome (AIDS). Hepatology. 1987;7(5):925–30. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura T, Mizuno S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86(6):588–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mercader M, Taddeo B, Panella JR, et al. Induction of HHV-8 lytic cycle replication by inflammatory cytokines produced by HIV-1-infected T cells. Am J Pathol. 2000;156(6):1961–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammerman AM, Kotner LM, Jr, Doyle TB. Periportal contrast enhancement on CT scans of the liver. AJR Am J Roentgenol. 1991;156(2):313–5. [DOI] [PubMed] [Google Scholar]
- 13.Valls C, Cañas C, Turell LG, et al. Hepatosplenic AIDS-related Kaposi's sarcoma. Gastrointest Radiol. 1991;16(4):342–4. [DOI] [PubMed] [Google Scholar]
- 14.Krown SE, Metroka C, Wernz JC. Kaposi's sarcoma in the acquired immune deficiency syndrome: A proposal for uniform evaluation, response, and staging criteria. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol. 1989;7(9):1201–7. [DOI] [PubMed] [Google Scholar]
- 15.Gbabe OF, Okwundu CI, Dedicoat M, et al. Treatment of severe or progressive Kaposi's sarcoma in HIV-infected adults. Cochrane Database Syst Rev. 2014;9:CD003256. [DOI] [PubMed] [Google Scholar]
- 16.Volkow P, Cesarman-Maus G, Garciadiego-Fossas P, et al. Clinical characteristics, predictors of immune reconstitution inflammatory syndrome and long-term prognosis in patients with Kaposi sarcoma. AIDS Res Ther. 2017;14(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu CL, Chang SY, Tseng YT, et al. Immune reconstitution inflammatory syndrome of Kaposi's sarcoma in an HIV-infected patient. J Microbiol Immunol Infect. 2013;46(4):30. [DOI] [PubMed] [Google Scholar]