Fig. 1. Design of WDR5 degrader MS33 and the crystal structure of the VCB-MS33-WDR5 ternary complex.
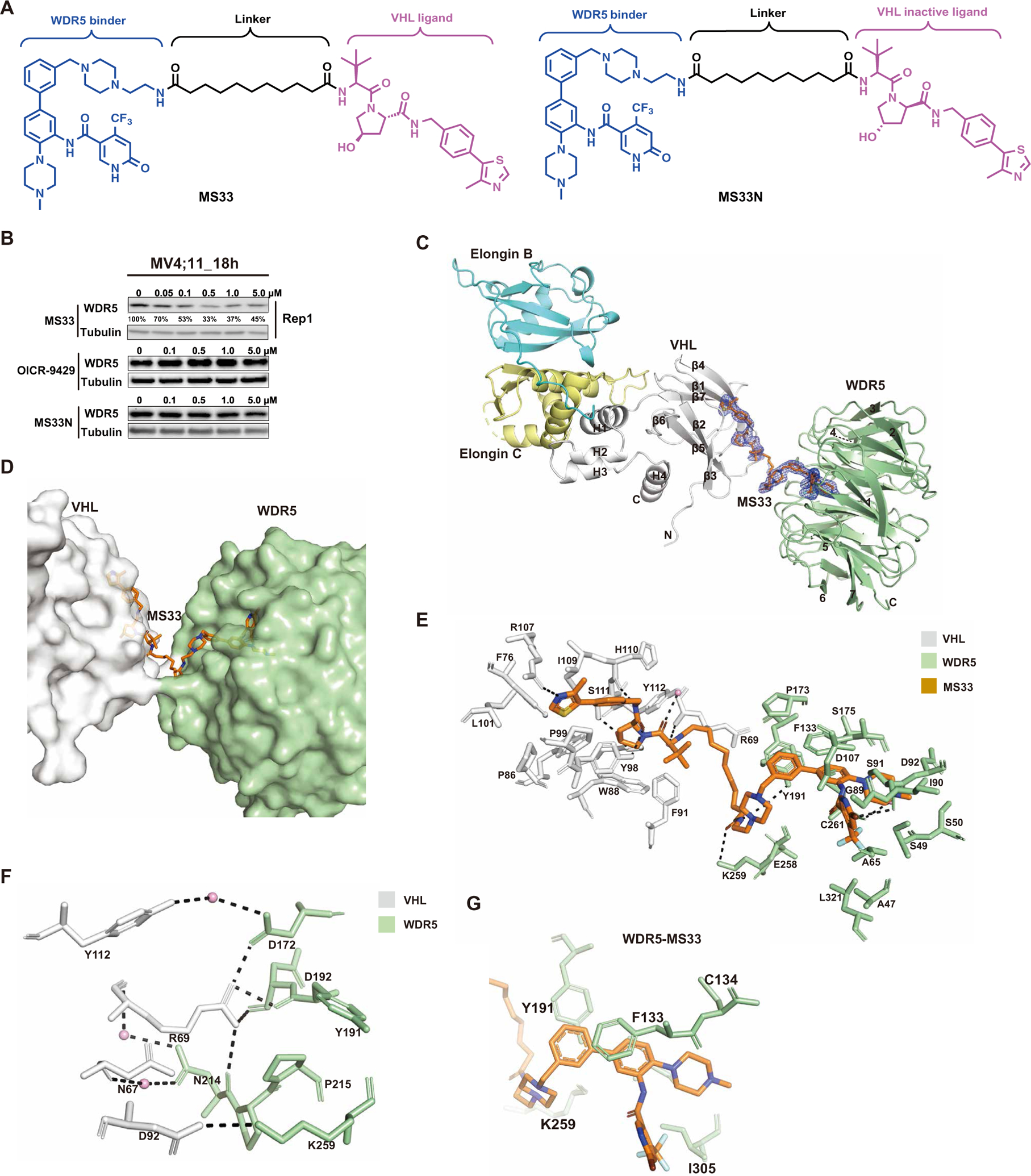
(A) Chemical structures of WDR5 degrader MS33 and a negative control of MS33, MS33N. (B) Immunoblots for WDR5 and Tubulin posttreatment of MV4;11 cells with the indicated concentrations of MS33, OICR-9429, or MS33N for 18 hours. (C) Overall structure of the VCB-MS33-WDR5 ternary complex displayed in ribbon representation with VHL, Elongin C, Elongin B, and WDR5 colored in gray, pale yellow, cyan, and pale green, respectively. The secondary structure elements for VHL are labeled. The seven β-propellers of WDR5 are also labeled. The simulated annealing Fo-Fc omit map (blue mesh) for MS33 is displayed (contoured at 3.0σ with a carve radius of 2.0 Å). (D) Overview of the VHL-MS33-WDR5 ternary complex, with VHL, WDR5, and MS33 shown in gray, pale green, and orange, respectively. (E) Detailed view of the binding interactions of MS33 with VHL (gray) and WDR5 (pale green) in the VCB-MS33-WDR5 complex. Only amino acids within 4-Å spheres of MS33 are depicted. Water molecules are depicted as pink spheres. Hydrogen bonds are depicted by dashed lines. (F) VHL-WDR5 interface in the VCB-MS33-WDR5 complex. The key amino acids participating in interactions at the interface between VHL and WDR5 in the VCB-MS33-WDR5 complex are shown. Arg69 exists in two conformations, and only one conformation is shown for clarity. Water molecules are depicted as pink spheres. Hydrogen bonds are depicted by dashed lines. (G) Close-up view of contacts between the WDR5 binding moiety of MS33 (in orange) and WDR5 residues (in pale green) in the VCB-MS33-WDR5 ternary complex.
