Fig. 7. MS67 is efficacious in vivo in a MLL-r AML xenograft model.
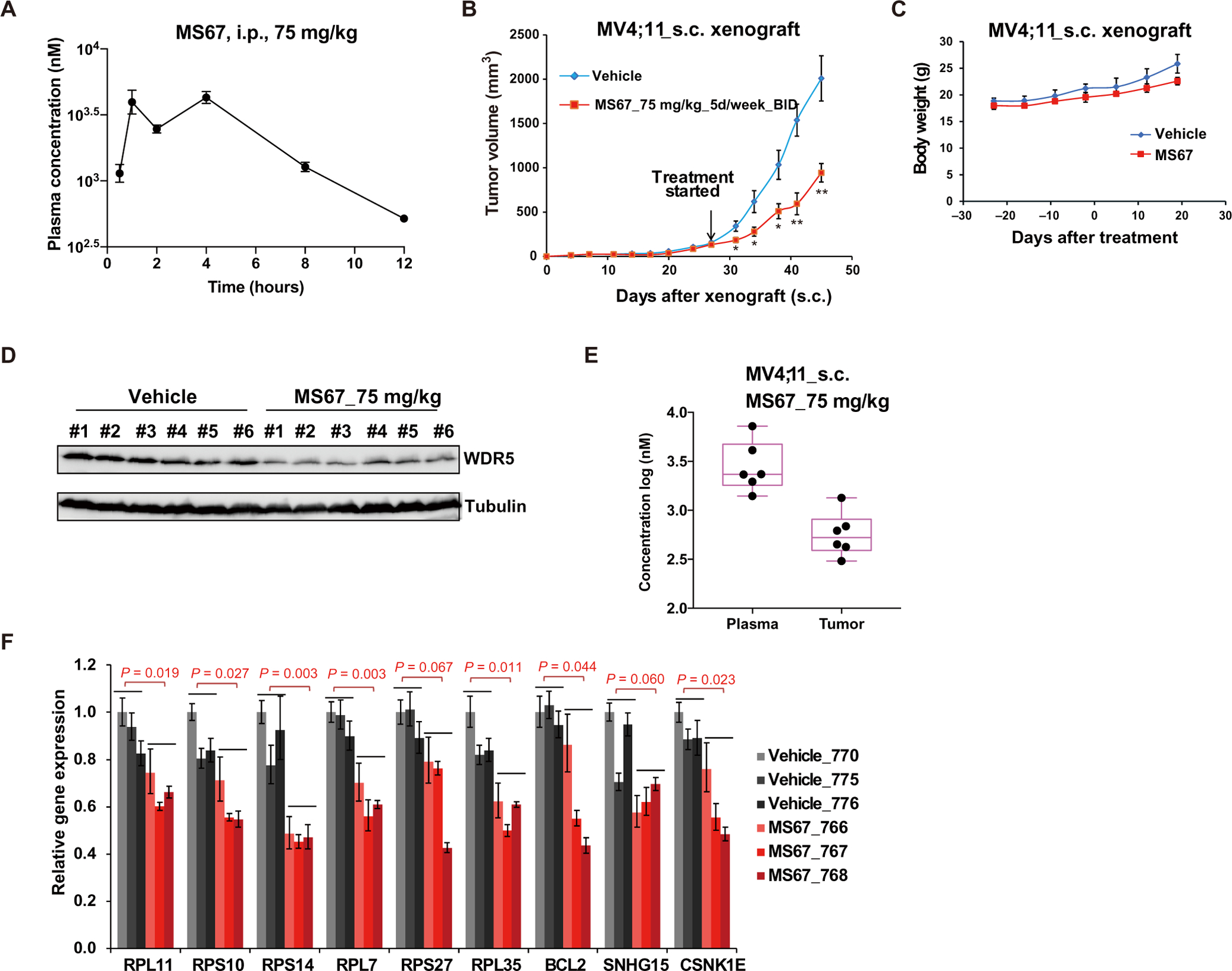
(A) Plasma concentrations of MS67 over a 12-hour period in mice after a single intraperitoneal (i.p.) injection of MS67 (75 mg/kg). The plasma concentrations represent the means ± SEM from three mice per time point. (B) The effect of MS67 treatment on the growth of MV4;11 tumors xenografted subcutaneously (s.c.). Tumor-bearing NOD/SCID/gamma(c)(null) (NSG) mice were treated with either vehicle (blue; n = 8) or MS67 [75 mg/kg, i.p. twice daily (BID); red; n = 10] for 5 days per week, starting at day 26 after inoculation. Y axis shows the tumor volumes, measured every 2 to 3 days and presented in the means ± SEM. Student’s t test, *P < 0.05 and **P < 0.01. (C) Body weights of NSG mice bearing MV4;11 tumor xenografts, treated with either vehicle (blue; n = 5) or MS67 (75 mg/kg, i.p. BID; red; n = 5) for 5 days per week. (D) Immunoblots for WDR5 and Tubulin in tumor samples isolated from NSG mice bearing MV4;11 tumor xenografts. Tumor samples were collected at 2 hours after the last dose from NSG mice treated with vehicle (left) or MS67 (75 mg/kg, i.p. BID; right) for five consecutive days. (E) MS67 concentrations in plasma (left) and tumor samples (right) isolated from six NSG mice bearing MV4;11 tumor xenografts. Tumor and plasma samples were collected at 2 hours after the last dose from NSG mice treated with MS67 (75 mg/kg, i.p. BID) for five consecutive days. (F) RT-qPCR for the indicated WDR5 target genes in tumor samples isolated from NSG mice bearing MV4;11 tumor xenografts. Tumor samples were collected at 2 hours after the last dose from NSG mice treated with vehicle (gray, n = 3) or MS67 (75 mg/kg, i.p. BID; red) for five consecutive days.
