Fig. 3. Allele-specific suppression of a PPP1R15A surface-charge mutation by a reciprocal surface-charge mutation in β-actin.
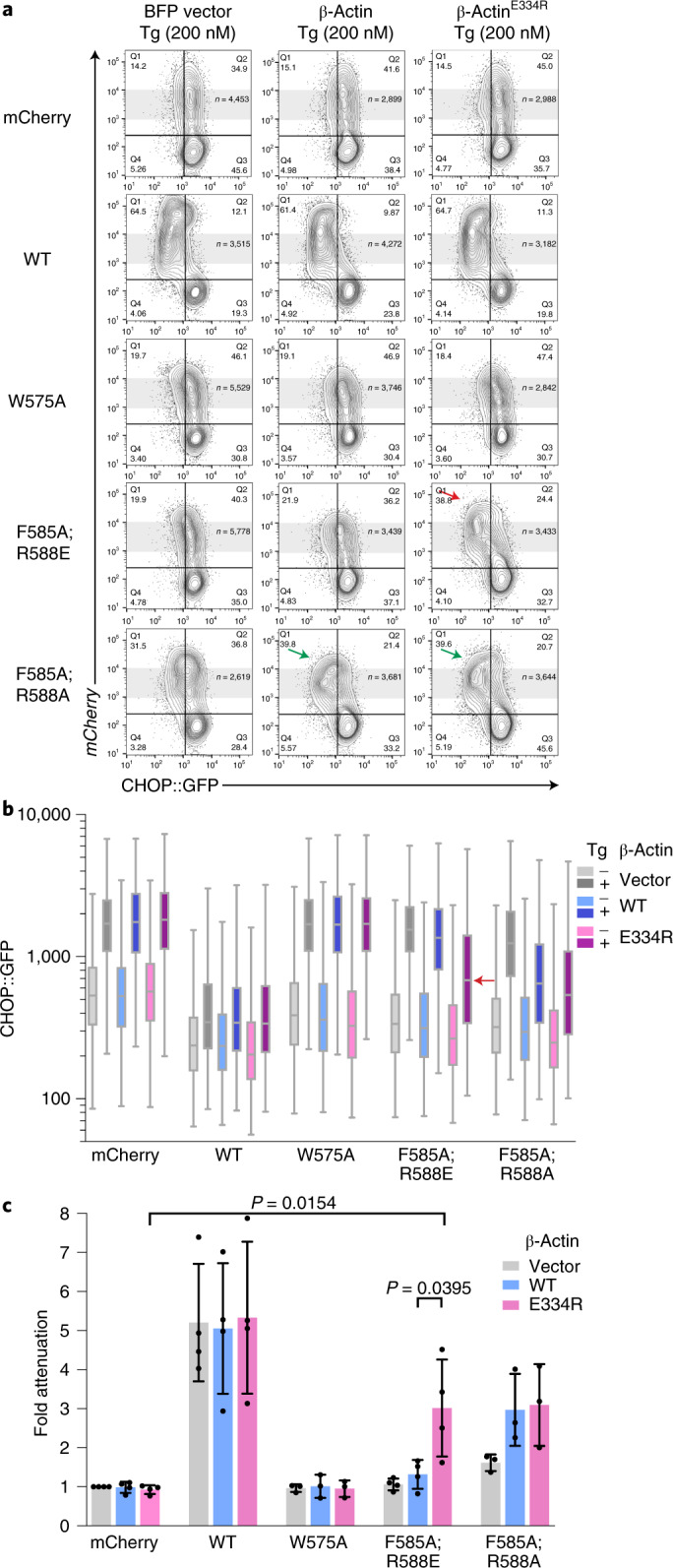
a, Two-dimensional flow cytometry dot plots of thapsigargin-treated cells transfected with plasmids encoding either blue fluorescent protein (BFP) alone, BFP linked (in-trans) to an otherwise wild-type (polymerization-deficient A204E;P243K) β-actin or BFP linked to polymerization-deficient β-actinE334R surface-charge reversal mutant (affecting a residue that forms a salt bridge with mouse PPP1R15AR588). Cells were cotransfected with plasmids encoding mCherry alone or the indicated mouse PPP1R15A::mCherry fusion proteins (as in Fig. 2e–g). Shown are the mCherry and GFP channels of the β-actin-expressing (BFP+) populations in each dataset. β-ActinE334R selectively restores the ability of the PPP1R15AF585A;R588E mutation to attenuate the ISR (red arrow), whereas both the wild type and β-actinE334R enhance ISR attenuation by PPP1R15AF585A;R588A (green arrows). b, Box (25th and 75th percentile) and whiskers plots (cut-off at the 1–99th percentiles) of the CHOP::GFP signal in untreated (–) and thapsigargin-treated (+) from the BFP+, mCherry+ cells marked by gray shading in a above (cell numbers shown in a). The signal in the critical sample coexpressing PPP1R15AF585A;R588E and β-actinE334R is denoted by a horizontal red arrow. c, Individual data points and mean ± s.d. of the ISR attenuation factor of the indicated subset of PPP1R15A::mCherry fusions from replicate experiments as in a (P values for two-tailed t-test comparisons shown, n = 4 independent experiments for mCherry, WT and F585A;R588E and n = 3 for W575A and F585A;R588A). Source data for a–c are available online.
