Abstract
Objectives:
Hypertension is a risk factor for chronic kidney disease (CKD) progression and mortality. However, the optimal blood pressure associated with decreased mortality in each stage of CKD remains uncertain.
Methods:
In this retrospective cohort study, we included 13,414 subjects with CKD stages 1–4 from NHANES general population datasets from 1999–2004 followed to 12/31/2010. Multivariate analysis and Kaplan-Meier curves were used to assess systolic blood pressure (SBP) and risk factors associated with overall mortality in each CKD stage.
Results:
In these subjects with death rates of 9%, 12%, 30%, and 54% in baseline CKD stages 1 through 4, respectively, SBP less than 100 mmHg was associated with significantly increased mortality adjusted for age, sex, and race in stages 2,3,4. After excluding SBP <100 mmHg, as a continuous variable, higher SBP is associated with fully adjusted increased mortality risk in those on or not on antihypertensive medication (HR 1.006, P=0.0006 and HR 1.006 per mmHg, P<0.0001, respectively). In those on antihypertensive medication, SBP <100 mmHg or in each 20 mmHg categorical group >120 mmHg is associated with an adjusted risk of increased mortality. Increasing age, males, smoking, diabetes, and comorbidities are associated with increased mortality risk.
Conclusions:
For patients with CKD stages 1–4, the divergence of SBP above or below 100–120 mmHg was found to be associated with higher all-cause mortality, especially in those patients on antihypertensive medication. These findings support the recent guideline of an optimal target goal SBP of 100–120 mmHg in patients with CKD stages 1 to 4.
Keywords: hypertension, chronic kidney disease, systolic blood pressure, mortality risk, stage 1–4 CKD
CONDENSED ABSTRACT
Hypertension is a risk factor for chronic kidney disease (CKD) progression and mortality. In this retrospective cohort study of 13,414 subjects with CKD from NHANES general population datasets, the death rates were 9%, 12%, 30%, and 54% in baseline CKD stages 1 through 4, respectively, over a mean follow-up of 71 months. Those with SBP<100 or with higher SBP>120 mm Hg were associated with higher all-cause mortality, especially in those patients on antihypertensive medication. These observational findings support the most recent guideline of an optimal target goal SBP of 100–120 mmHg in patients with CKD stages 1 to 4.
INTRODUCTION
Chronic kidney disease (CKD) is a significant and growing public concern. The prevalence of CKD among Medicare patients aged 65 or above was 14.5% in 2017, and the majority of them were stages 2 and 3.1 CKD is silent, and often unrecognized, until advanced stages. However, even early stages of CKD are associated with significant morbidity, mortality and reduction in quality of life.2,3 The relationship between hypertension and CKD is complex as hypertension may cause, aggravate, or complicate CKD.4 Blood pressure (BP) control has long been a cornerstone of care for patients with CKD, and more stringent BP targets have recently been recommended.5–7 As a recent study from the Chronic Renal Insufficiency Cohort showed, systolic blood pressure (SBP) of 140 mmHg and above is significantly associated with renal disease progression among CKD in the stage 3 to 5 population.8 Conversely, hypotension is common and concerning among CKD patients treated with antihypertensive drugs,9,10 and hypertension has also been reported to predict lesser end-stage kidney disease (ESKD) and mortality.11 The Kidney Disease Improving Global Outcomes (KDIGO) guidelines had previously recommended a BP target of 140/90 mmHg for patients with all stages of CKD and 130/80 mmHg for the subset of patients with proteinuria.12 However, the 2019 Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines recommend treatment to a goal BP of less than 130/80 mm Hg in CKD patients13 and the 2021 KDIGO guidelines now favor an SBP of less than 120 mm Hg.7
Since the optimal BP associated with the lowest all-cause mortality in patients with different stages of CKD remains unclear, the goal of this study was to identify the SBP and other risks factors that might predict all-cause mortality in the general population found to have CKD in stages 1 to 4 using a National Health and Nutrition Examination Survey (NHANES) dataset.
METHODS
Study population
In this retrospective analysis of NHANES data,14 files covering 1999–2000 (n=9,965), 2001–2002 (n=11,039), and 2003–2004 (n=10,122) were merged and variable name inconsistencies were corrected in the merged dataset. It is noted that data collection for NHANES was based on a substantial oversampling of young children, females, older persons, black persons, and Mexican Americans in order to identify those most at risk for poor nutrition for the purpose of the NHANES study. To provide the actual data, we used an unweighted analysis so that our NHANES group did not fully mirror percentages of a random sample of the U.S. population.15 Only adults (18 years old or older) with CKD stage 1 to 4 were included in our analysis. Presence and stage of CKD were defined based on estimated glomerular filtration rate (eGFR) calculated using the CKD-EPI expression16,17 (CKD stage 1: eGFR >90 with high urine albumin to creatinine ratio >30 mg/g; stage 2: eGFR 60–89; stage 3: eGFR 30–59; stage 4: eGFR 15–29 ml/min per 1.73 m2). Records with missing eGFR value or missing mortality information were deleted. Subjects with ESKD (Stage 5 CKD) were excluded. For the blood pressure data, the subjects first rested quietly in a sitting position for 5 minutes in the NHANES mobile examination center. After determining the maximum inflation level with a mercury sphygmomanometer, at least three, and sometimes 4, BP determinations were taken by a trained examiner. We used the average of all SBP measurements for this study. Since only unidentified patient data were analyzed, the hospital institutional review board determined that this study did constitute human subjects research and did not require IRB approval.
Outcome variable
The outcome in this study is all-cause mortality. The mortality information was obtained from the Centers for Disease Control and Prevention (CDC) website and linked to the NHANES data using the unique subject ID. The National Center for Health Statistics has conducted a mortality linkage of NHANES to death certificate data found in the National Death Index. The NHANES Linked Mortality Files include the continuous NHANES years (1999–2010) and provide mortality follow-up data from the date of survey participation through December 31, 2010.
Covariates
In primary prevention, the association of BP-lowering treatment with major cardiovascular events was dependent on baseline SBP.18 Moreover, isolated diastolic hypertension was not significantly associated with increased risk for cardiovascular outcomes,19 and lower diastolic BP was associated with increased risk of all-cause mortality,20 but the benefit of intensive SBP lowering remained consistent across the baseline diastolic BP range in the Systolic Blood Pressure Intervention Trial (SPRINT).20 Therefore, our primary variable of interest is SBP analyzed as a continuous variable and divided into six categories of 20 mm Hg each starting at 100 mm Hg.
Variables considered to have potential confounding effect were included in the multivariate models, specifically demographics of the subjects (i.e., age, sex, race); use of antihypertensive medications (binary variable), history of diabetes mellitus, history of smoking, hyperlipidemia, body mass index (BMI), and number of comorbidities which included presence of liver disease, coronary artery disease, history of stroke, chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), cancer, anemia, hyperphosphatemia, and significant hyperkalemia (defined as a serum potassium level >6.0 mEq/L).
Statistical analyses
Means and standard deviations were used to summarize continuous variables with normal distributions. Categorical variables were summarized as percent of total. The data collected were analyzed using SAS software version 9.3 (SAS Institute, Cary, North Carolina).
Multivariate analysis was performed using a proportional hazard model that is not time dependent. As described, subjects were accrued from 1999 to 2004 and only censored for the outcome of death and the end of the study (December 31, 2010). Survival was calculated using PERMTH_EXM variable (person-months of follow-up), which is the number of months from examination date through the date of death, where applicable. For respondents assumed alive, person-months of follow-up were calculated from examination date through the end of the mortality follow-up period, December 31, 2010. We also performed subgroup analysis based on stage of CKD, and presence of antihypertensive medication treatment. When specific covariate data was missing, the subject was omitted from that subgroup analysis.
RESULTS
Baseline characteristics of the study population
Out of a total of 31,126 NHANES participants, we included 13,414 subjects with CKD stages 1–4 (43.1%) in our analysis (Table 1). The mean age was 59.8 years, 51.3% were males and 22.7% were non-Hispanic black. Stage 1 CKD, defined as eGFR >90 ml/min/1.73m2 with albuminuria, presented in 9.9% of the subjects with CKD; stages 2, 3, and 4 presented in 75.2%, 14.0% and 1.0% of the CKD population, respectively. Of the study population 19.5 % had diabetes mellitus, 50.7% had hypertension based on measured blood pressure and self-identified questionnaire, and 36.0% were taking antihypertensive medication. However, only 41.1% of the population were aware of the diagnosis of hypertension by questionnaire. During this 12 year study period, the death rates were 15.4% overall with 9.0%, 11.9%, 30.2%, and 54.1% in CKD stages 1 through 4, respectively, over a mean duration of follow-up of 71.0 months (95% CI 68.9–73.1 months) (Table 1 and Figures 1A–D).
Table 1.
Baseline characteristics of the NHANES study population with CKD stages 1–4
| Total N = 13,414 |
CKD stage 1 N = 1,428 (9.9%) |
CKD stage 2 N = 9,306 (75.2%) |
CKD stage 3 N = 2,471 (14.0%) |
CKD stage 4 N = 209 (1.0%) |
|
| Age in years (SD) | 59.8 (17.4) | 42.1 (16.7) | 53.7 (16.1) | 73.1 (10.3) | 74.6 (11.0) |
| Sex (%) Male Female |
6885 (51.3) 6529 (48.7) |
558 (39.1) 870 (60.9) |
5067 (54.5) 4239 (45.6) |
1164 (47.1) 1307 (52.9) |
96 (45.9) 113 (54.1) |
| Race (%) Non-Hispanic White Non-Hispanic Black Hispanic Other |
7464 (55.6) 3039 (22.7) 2487 (18.5) 424 (3.2) |
489 (34.2) 263 (18.4) 599 (42.0) 77 (5/4) |
5332 (57.3) 2103 (22.6) 1588 (17.1) 283 (3.0) |
1529 (61.9) 613 (24.8) 276 (11.2) 53 (2.1) |
114 (54.6) 60 (28.7) 24 (11.5) 11 (5.3) |
| Deaths over mean 71 months of follow-up (%) | 2093 (15.4) | 129 (9.0) | 1104 (11.9) | 747 (30.2) | 113 (54.1) |
| Systolic blood pressure in mmHg (SD) | 131.1 (21.9) | 127.3 (22.4) | 129.7 (20.7) | 137.7 (23.8) | 143.0 (29.6) |
| Diastolic blood pressure in mmHg (SD) | 69.7 (15.2) | 72.4 (14.4) | 70.9 (14.0) | 64.4 (17.7) | 61.6 (19.6) |
| Presence of diabetes (%) | 2610 (19.5) | 404 (28.3) | 1384 (14.9) | 731 (29.6) | 91 (43.5) |
| Smoking history (%) | 1992 (14.9) | 316 (22.1) | 1459 (15.7) | 201 (8.1) | 16 (7.7) |
| eGFR in mL/min/1.73 m2 (SD) | 75.0 (19.5) | 110.2 (14.7) | 77.5 (8.4) | 49.5 (7.8) | 24.4 (4.1) |
| Urine albumin-to-creatinine ratio (mg/g)* (SD) | 79.8 (481.0) | 186.4 (674.6) | 35.3 (209.0) | 127.4 (641.7) | 816.4 (2126.5) |
| BMI* (SD) | 28.8 (6.3) | 29.1 (7.5) | 28.7 (6.1) | 29.1 (6.4) | 29.5 (6.7) |
| Comorbidities – None (%) | 6330 (47.2) | 801 (56.09) | 4760 (51.2) | 750 (30.4) | 19 (9.1) |
| Comorbidities – 1 (%) | 4382 (32.7) | 446 (31.2) | 3038 (32.7) | 837 (33.9) | 61 (29.2) |
| Comorbidities – 2 (%) | 1785 (13.3) | 137 (9.6) | 1059 (11.4) | 539 (21.8) | 50 (23.9) |
| Comorbidities – 3 (%) | 618 (4.6) | 35 (2.5) | 323 (3.5) | 213 (8.6) | 47 (22.5) |
| Comorbidities – 4 (%) | 220 (1.6) | 8 (0.6) | 91 (1.0) | 99 (4.0) | 22 (10.5) |
| Comorbidities – 5 (%) | 79 (0.6) | 1 (0.1) | 35 (0.4) | 33 (1.3) | 10 (4.8) |
There were 347 subjects with missing data for urine albumin to creatinine ratio and 472 subjects with missing data for BMI.
Figure 1.

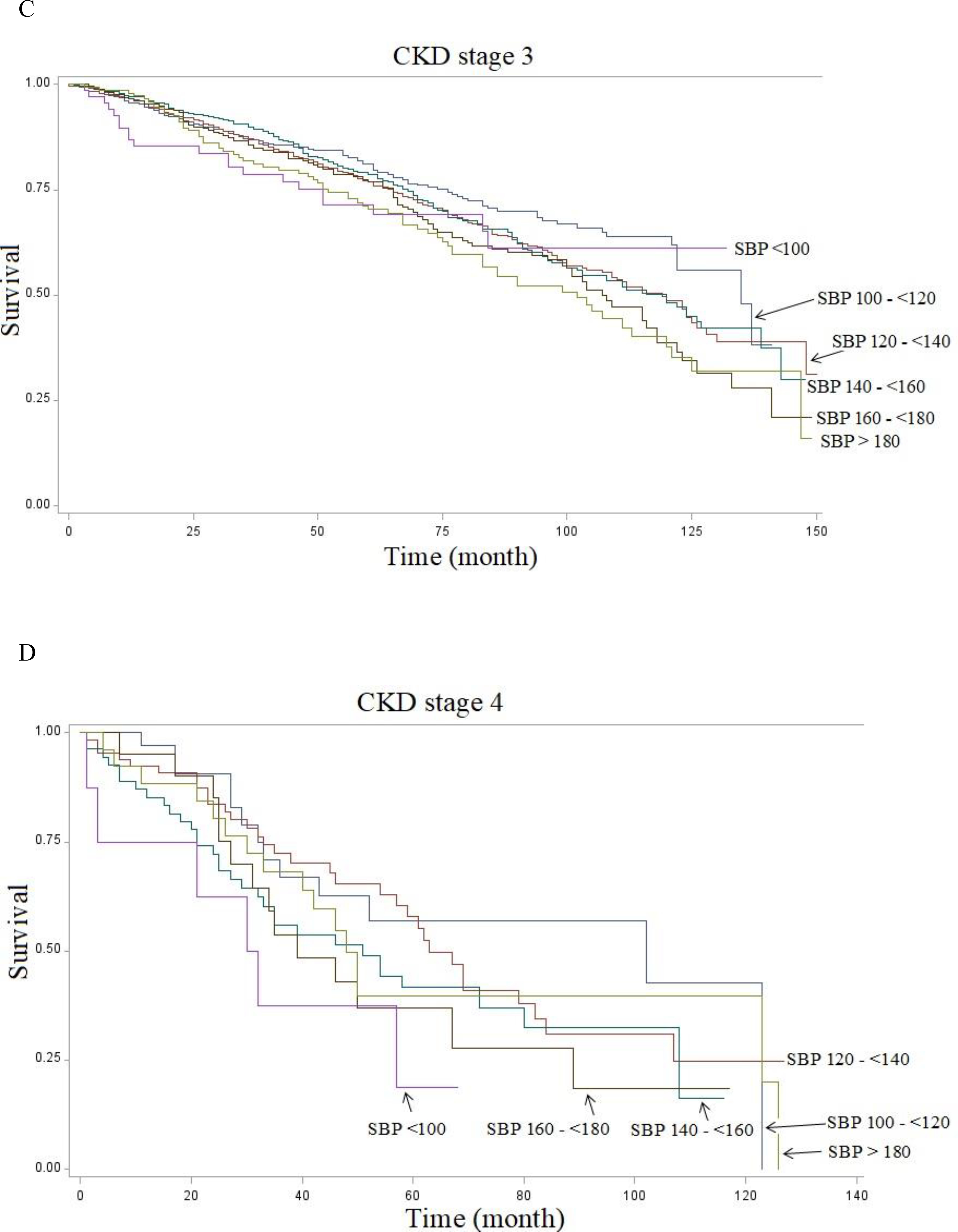
Kaplan-Meier Curves of survival of 13,414 NHANES subjects with CKD followed from 1999 to 2010 shown by systolic blood pressure (SBP) levels at 20 mmHg intervals. A. Stage 1: 1428 patients. B. Stage 2: 9306 patients. C. Stage 3: 2471 patients. D. Stage 4: 209 patients.
Multivariate analysis
In multivariate analysis adjusted for only age, sex and race, SBP less than 100 mm Hg was associated with increased mortality in individuals with baseline CKD stages 2, 3 and 4, respectively (HR 1.57 CI 1.21–2.06, P=0.03; HR 1.80, CI 1.38–2.48, P=0.01; HR 4.03 CI 1.21–5.19, P=0.003, Table 2, Model 1), whereas significance was lost when adjusted for all risk covariates in stages 2 and 3 (Table 2, Model 2). As a categorical variable, none of the three 20-mmHg categories with SBP between 100 and 160 mm Hg showed statistically significant differences in mortality in all CKD stages. SBP 160–180 had increased mortality risk in CKD stage 2 after adjusting for all risk covariates (HR 1.44, P=0.002). SBP>180 was associated with increased mortality in individuals with baseline CKD stage 1 and 2 when adjusted for age, sex and race or for all risk covariates (Table 2, Model 1/Model 2 with HR 2.75/HR 2.46, P=0.007/P=0.02; HR 1.39/HR 1.50, P=0.03/P=0.01, respectively). Given the “J” shape effect of SBP on mortality, we excluded the population of patients with SBP<100 when using SBP as a continuous variable because of their increased mortality in order to assess the risk of hypertension.
Table 2.
Results of risk factors for mortality by multiple regression adjusted for age, gender and race (Model 1) and all risk factors (Model 2) in CKD stages 1–4 with systolic blood pressure (SBP) as a categorical variable.
| CKD Stage 1 | CKD Stage 2 | CKD Stage 3 | CKD Stage 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Model 1 (N = 1428) | Model 2 (N = 1402)* | Model 1 (N = 9306) | Model 2 (N= 9149)* | Model 1 (N = 2471) | Model 2 (N= 2367)* | Model 1 (N = 209) | Model 2 (N = 192)* | |
| Hazard Ratio (P) | Hazard Ratio (P) | Hazard Ratio (P) | Hazard Ratio (P) | Hazard Ratio (P) | Hazard Ratio (P) | Hazard Ratio (P) | Hazard Ratio (P) | |
| SBP<100 (N=491) | 2.04 (0.26) | 1.94 (0.29) | 1.57 (0.03) | 1.48 (0.07) | 1.80 (0.01) | 1.38 (0.21) | 4.03 (0.003) | 3.22 (0.06) |
| SBP 100–120 (N=4027) | Reference | |||||||
| SBP 120–140 (N=4973) | 1.35 (0.32) | 1.33 (0.35) | 0.91 (0.32) | 1.01 (0.92) | 1.11 (0.35) | 1.12 (0.35) | 1.37 (0.34) | 1.49 (0.3) |
| SBP 140–160 (N=2530) | 1.53 (0.18) | 1.42 (0.29) | 0.97 (0.77) | 1.13 (0.24) | 0.94 (0.63) | 0.99 (0.93) | 1.82 (0.07) | 1.96 (0.08) |
| SBP 160–180 (N=943) | 0.95 (0.98) | 0.93 (0.85) | 1.25 (0.05) | 1.44 (0.002) | 1.14 (0.35) | 1.13 (0.43) | 1.94 (0.09) | 1.63 (0.29) |
| SBP>180 (N=450) | 2.75 (0.007) | 2.46 (0.02) | 1.39 (0.02) | 1.50 (0.01) | 1.21 (0.21) | 1.36 (0.06) | 1.40 (0.38) | 1.25 (0.63) |
| Age (per year) | 1.07 (<0.0001) | 1.07 (<0.0001) | 1.10 (<0.001) | 1.09 (<0.0001) | 1.08 (<0.001) | 1.08 (<0.0001) | 1.05 (<0.0001) | 1.05 (<0.001) |
| Sex (Female) | 0.46 (<0.0001) | 0.46 (0.0002) | 0.61 (<0.001) | 0.68 (<0.0001) | 0.68 (<0.001) | 0.71 (<0.0001) | 0.94 (0.74) | 1.25 (0.37) |
| Race (Black) | 0.61 (0.08) | 0.57 (0.06) | 1.42 (0.0001) | 1.27 (0.01) | 1.04 (0.69) | 0.99 (0.89) | 1.16 (0.53) | 1.24 (0.42) |
| Anti-hypertensive medication | 1.42 (0.09) | 1.05 (0.47) | 0.99 (0.92) | 1.34 (0.31) | ||||
| eGFR (per 1 ml/min /1.73m2) | 1.01 (0.68) | 0.99 (<0.05) | 0.98 (<0.001) | 0.95 (0.06) | ||||
| Smoking | 1.76 (0.005) | 2.12 (<0.0001) | 1.71 (0.0014) | 1.24 (0.57) | ||||
| Diabetes | 1.16 (0.49) | 1.33 (<0.001) | 1.34 (0.0004) | 0.95 (0.84) | ||||
| Hyper-lipidemia | 1.25 (0.28) | 0.80 (<0.001) | 0.87 (0.09) | 0.76 (0.26) | ||||
| BMI (per 1 Kg/m2) | 0.96 (0.01) | 0.99 (0.04) | 0.997 (0.71) | 0.96 (0.09) | ||||
| Comorbidities (number) 1 | 1.47 (0.06) | 1.41 (<0.0001) | 1.20 (0.08) | 1.13 (0.78) | ||||
| Comorbidities 2 | 1.55 (0.14) | 1.94 (<0.0001) | 1.43 (0.002) | 0.96 (0.92) | ||||
| Comorbidities 3 | 2.35 (0.04) | 2.16 (<0.0001) | 1.77 (<0.0001) | 1.76 (0.23) | ||||
| Comorbidities 4 | 4.17 (0.06) | 3.99 (<0.0001) | 2.89 (<0.0001) | 1.22 (0.73) | ||||
| Comorbidities 5 | 0.000** (<0.0001) | 3.54 (<0.0001) | 3.05 (<0.0001) | 1.94 (0.24) | ||||
The total number of subjects for Model 2 is slightly lower than Model 1 because of subjects that were missing data for adjustment variables.
This apparent ‘paradox’ of decreased mortality with 5 comorbidities is likely because there was only one subject with 5 comorbidities in Stage 1 CKD.
Using SBP as a continuous variable (excluding SBP<100), after adjusting for all risk covariates, every 10 mm Hg increase above 100 mm Hg is associated with 6% (P<0.0001) increased mortality risk in CKD stage 2 (Table 3). Every 10 mm Hg increase in SBP above 100 mm Hg is also associated with 10% increased mortality risk in CKD stage 1, 2% in stage 3 and 10% in stage 4, although those did not reach statistical significance (P=0.11, P=0.15, and P =0.09, respectively).
Table 3.
Results of adjusted multiple regression model of all risk factors for mortality by CKD stage with systolic blood pressure (SBP) as a continuous variable with subjects with SBP<100 mm Hg excluded because of its opposite effect on mortality (see Table 1)
| CKD stage 1 (n=1,310)* | CKD stage 2 (n=8,829)* | CKD stage 3 (n=2,301)* | CKD stage 4 (n=186)* | |
|---|---|---|---|---|
| Hazard Ratio (P) | Hazard Ratio (P) | Hazard Ratio (P) | Hazard Ratio (P) | |
| SBP (per 1 mm Hg above 100 mm Hg) | 1.01 (0.11) | 1.006 (<0.0001) | 1.002 (0.15) | 1.01 (0.09) |
| Age (per year) | 1.07 (<0.0001) | 1.09 (<0.0001) | 1.08 (<0.0001) | 1.05 (0.003) |
| Sex (Female) | 0.47 (<0.0001) | 0.67 (<0.0001) | 0.72 (<0.0001) | 1.14 (0.59) |
| Race (Black) | 0.56 (0.057) | 1.25 (0.018) | 0.96 (0.68) | 1.16 (0.58) |
| Antihypertensive medication | 1.34 (0.16) | 1.05 (0.44) | 0.96 (0.64) | 1.24 (0.47) |
| eGFR (per 1 ml/min/1.73m2) | 1.01 (0.57) | 0.99 (0.061) | 0.98 (<0.0001) | 0.96 (0.17) |
| Smoking | 1.73 (0.008) | 2.15 (< 0.0001) | 1.88 (<0.0001) | 1.1 (0.80) |
| Diabetes | 1.16 (0.46) | 1.31 (0.003) | 1.34 (0.0019) | 0.96 (0.88) |
| Hyperlipidemia | 1.26 (0.27) | 0.79 (0.0006) | 0.88 (0.12) | 0.70 (0.15) |
| BMI (per 1 Kg/m2) | 0.95 (0.005) | 0.99 (0.026) | 0.998 (0.819) | 0.98 (0.26) |
| Comorbidities (number) 1 | 1.49 (0.06) | 1.42 (<0.0001) | 1.22 (0.065) | 1.17 (0.71) |
| Comorbidities 2 | 1.64 (0.09) | 1.94 (<0.0001) | 1.44 (0.002) | 1.12 (0.8) |
| Comorbidities 3 | 2.50 (0.032) | 2.19 (<0.0001) | 1.80 (<0.0001) | 2.16 (0.1) |
| Comorbidities 4 | 3.08 (0.13) | 3.87 (<0.0001) | 2.67 (<0.0001) | 1.08 (0.91) |
| Comorbidities 5 | NA** | 3.60 (<0.0001) | 3.22 (<0.0001) | 2.19 (0.17) |
The total number of subjects is less than 13,414 because of eliminating subjects with SBP<100 mm Hg and those with missing data for adjustment variables.
Analysis cannot be performed as there was only one subject with 5 comorbidities in Stage 1 CKD.
Age was associated with increased mortality risk in CKD stages 1–4 whereas diabetes mellitus has significantly increased risk in CKD stage 2 and 3. Smoking and male sex had an association with increased mortality risk in all CKD stages except stage 4 which only had 186 patients. Black race appeared to be protective only in CKD stage 1, although that did not quite reach statistical significance. Though hyperlipidemia was negatively associated with mortality in CKD stage 2, this analysis was not adjusted for anti-lipidemic medication use, which might have confounded the results. BMI had a mild protective effect on mortality in all CKD stages, a finding similar to its protective effect in dialysis patients21, but which reached statistical significance only in CKD stages 1 and 2 (HR 0.95, P=0.005; HR 0.99, P=0.026)
In subgroup analysis based on antihypertensive medication treatment versus no medication with SBP as a categorical variable, after adjusting for all risk factors (Table 4A), SBP 160–180 mm Hg and >180 mmHg are associated with increased mortality risk in patients on no antihypertensive medication. Whereas in patients taking antihypertensive medication treatment, SBP levels <100 or >140 mm Hg are all associated with significantly increased mortality risk when compared to those with SBP 100–120 mm Hg.
Table 4A.
Results of adjusted regression model of risk factors for mortality in entire CKD population (Stages 1–4) based on antihypertensive treatment vs no treatment with systolic blood pressure (SBP) as a categorical variable.
| Fully adjusted Model of patients not taking antihypertensive medications (N = 7,662)* | Fully Adjusted Model of patients taking antihypertensive medications (N = 5,448)* | |
|---|---|---|
| Hazard Ratio (P) | Hazard Ratio (P) | |
| SBP<100 | 1.26 (0.28) | 1.90 (0.003) |
| SBP 100–120 | Reference | |
| SBP 120–140 | 0.97 (0.75) | 1.21 (0.061) |
| SBP 140–160 | 1.01 (0.92) | 1.27 (0.02) |
| SBP 160–180 | 1.34 (0.026) | 1.42 (0.004) |
| SBP>180 | 1.67 (0.002) | 1.65 (0.0002) |
| Age (per year) | 1.09 (<0.0001) | 1.07 (<0.0001) |
| Sex (Female) | 0.70 (<0.0001) | 0.7 (<0.0001) |
| Race (Black) | 1.08 (0.51) | 1.06 (0.44) |
| eGFR (per 1 ml/min/1.73m2) | 0.997 (0.24) | 0.99 (<0.0001) |
| Smoking | 2.30 (<0.0001) | 1.75 (<0.0001) |
| Diabetes | 1.46 (<0.0001) | 1.28 (0.0003) |
| Hyperlipidemia | 0.82 (0.006) | 0.87 (0.037) |
| BMI (per 1 Kg/m2) | 0.97 (0.0005) | 0.99 (0.20) |
| Comorbidities (number) 1 | 1.46 (<0.0001) | 1.30 (0.002) |
| Comorbidities 2 | 1.82 (<0.0001) | 1.67 (<0.0001) |
| Comorbidities 3 | 2.12 (<0.0001) | 2.14 (<0.0001) |
| Comorbidities 4 | 4.55 (<0.0001) | 2.94 (<0.0001) |
| Comorbidities 5 | 7.07 (<0.0001) | 2.85 (<0.0001) |
After excluding SBP<100 mm Hg, using SBP as a continuous variable, SBP is associated with an increased mortality risk in both those not on antihypertensive medication and on antihypertensive medication treatment (HR 1.006, P=0.0006 and 1.006 per mm Hg increase, P<0.0001, respectively, Table 4B). It should be noted that there was no association of mortality risk with the use of antihypertensive medications in CKD stages 1–4 (Tables 2 and 3). Female sex and hyperlipidemia are protective factors whether on or off antihypertensive medication whereas smoking, diabetes, and number of comorbidities were associated with increased mortality risk in both groups (Table 4A).
Table 4B.
Results of adjusted regression model for mortality in entire CKD population (Stages 1–4) based on antihypertensive treatment vs no treatment with systolic blood pressure (SBP) as a continuous variable (SBP<100 was excluded because of its opposite effect on mortality-see Table 1). Model was adjusted based on SBP, age, gender, race, eGFR, smoking, diabetes, hyperlipidemia, BMI, and number of comorbidities.
| Fully adjusted Model of patients not taking antihypertensive medications (N - 7299)* | Fully Adjusted Model of patients taking antihypertensive medications (N = 5327)* | |
|---|---|---|
| Hazard Ratio (P) | Hazard Ratio (P) | |
| SBP (per mm Hg above 100 mm Hg) | 1.006 (0.0006) | 1.006 (<0.0001) |
The total number of subjects is less than 13,414 because of subjects with missing data for adjustment variables.
As shown in the Kaplan-Meier curves, the SBP groups above 120 or below 100 mm Hg are associated with increased mortality in each CKD stage (Figure 1) or whether on antihypertensive medication treatment or not (Figure 2).
Figure 2.

Kaplan-Meier Curves of survival of 13,414 NHANES subjects with CKD followed from 1999 to 2010 shown by systolic blood pressure (SBP) levels at 20 mmHg intervals based on antihypertensive medication usage. A. No anti-hypertensive medication, 7299 patients*. B. On antihypertensive medication, 5327 patients*.
* The total number of subjects is less than 13,414 because of patients with missing data for adjustment variables.
DISCUSSION
Hypertension and CKD are highly prevalent, increasing worldwide, and present global public health challenges.22 Approximately one in three U.S. adults is affected by hypertension, while 6.5% overall and 14.5% of persons over 65 years are affected by CKD in stages 3–5.1,23–26 The prevention and control of hypertension is an essential component to reduce the progression and high mortality in the CKD population.
The SPRINT trial demonstrated that targeting SBP <120 mm Hg in comparison with <140 mm Hg was associated with reduction in mortality among patients at high risk for cardiovascular events but without diabetes.27 A meta-analysis of 42 randomized controlled trials demonstrated that lowering SBP to 120–124 mm Hg was associated with the lowest risk of cardiovascular outcome and mortality in adults treated with antihypertensive therapy.28 The most appropriate targets for SBP to reduce mortality among CKD patients remain uncertain. In the prespecified subgroup analyses for SPRINT participants with baseline CKD, intensive BP control with a SBP target of 120 mm Hg had a 28% lower rate of all-cause mortality compared with standard BP control with a SBP target of 140 mm Hg in mild to moderate CKD without diabetes during 3.3 years of follow-up.5 In black patients with hypertensive CKD and proteinuria, more aggressive blood pressure lowering was associated with renoprotection in patients with proteinuria only.29 A meta-analysis by Malhotra et al. showed that a more intensive SBP lowering regimen of 16 mm Hg vs 8 mm Hg from a baseline SBP of 140 mm Hg resulted in a 14% lower risk of all-cause mortality in patients with CKD stages 3 to 5. However, conclusions on specific BP goals could not be made because of heterogeneity in study designs.30 Nevertheless, knowledge of the benefits and harms of pursuing more intensive SBP goals below 120 mm Hg is particularly lacking, especially since concerns have been raised that intensive BP lowering might increase adverse clinical outcomes.31 In addition, the optimal BP goal for different CKD stages, particularly early stages of CKD, is not known. A recent review suggested a BP goal of <130/80 is a reasonable, evidence-based BP goal in patients with CKD.6 At this time KDIGO 2021 guidelines propose that “We suggest that adults with high BP and CKD be treated with a target systolic blood pressure (SBP) of <120 mm Hg, when tolerated, using standardized office BP measurement”.7 However, this recommendation may be considered to be “weak because potential benefits and harms may vary with CKD stage, diabetes, individuals with SBP 120–129, advanced CKD or the very old or frail”.32 Since the optimal SBP target for CKD populations remains unclear, we conducted this retrospective study to determine the association between SBP levels and mortality in CKD patients in each stage from 1 to 4.
Our study of data from NHANES subjects with CKD stages 1–4, having death rates from 9.0% to 54.1% over a mean duration of 71 months, showed that when the SBP exceeds 100 mm Hg, every 10 mm Hg increment of SBP is associated with a significantly increased risk of 6% for all-cause mortality in CKD stage 2 (with a similar but not significant trend in CKD stages 1, 3, and 4). These findings offer additional support for the lower BP target (i.e., SBP 100–120 mm Hg) espoused by the newest KDIGO guidelines7 to reduce all-cause mortality in the CKD patient population and show similar data for all stages 1– 4 CKD. However, SBP<100 mm Hg was associated with about a doubled risk of mortality when taking antihypertensive medications, suggesting a narrower blood pressure target window for safety in individuals taking blood pressure medication.
Our finding of the J-shaped relationship between SBP and mortality appears to confirm previous studies,33,34 although concomitant comorbid conditions may well contribute to the increased mortality in subjects with SBP<100 mm Hg. Since higher SBP levels above 100 mm Hg were associated with higher all-cause mortality, our study supports that more intensive BP lowering is associated with a mortality benefit in patients with early and advanced stages of CKD. One major innovative feature of this study is the use of a large nationwide survey database of an unselected general population to examine the association of ‘standardized office-measured’ SBP and all-cause mortality in participants found to have various CKD stages from 1 to 4. This study adds to the data that may inform healthcare professionals and policymakers of preferable BP treatment targets to help guide clinical practice until randomized controlled data become available.
Our study analysis has several limitations that deserve mention. In studies conducted in self-reported surveys, data analyses might be prone to misclassification bias or missing data. We do not have access to confirm antihypertensive medication use or identify possible causes of hypertension or hypotension. Second, we do not have access to relevant data on unmeasured confounders (i.e. duration of hypertension, antihypertensive medication class, BP fluctuations, nocturnal BP). These confounders could affect study SBP classification and all-cause mortality.35,36 Third, our study could not assess progressive kidney disease outcomes due to lack of longitudinal data though recent data seem to indicate that “patients with higher SBP (>120 mm Hg) had steeper slopes of eGFR decline”.37 Fourth, we could not assess possible symptoms associated with high or low BP or side effects of antihypertensive medications, which might affect clinical practice in managing BP. Fifth, since the relationship between SBP and mortality varied systematically with age in a CKD population,38 different age groups might benefit from different SBP targets. However, our observational data of properly measured standardized office BP levels confirms and extends the recent findings of increased mortality associated with increased SBP in the Chronic Renal Insufficiency Cohort (CRIC) Study of mainly CKD stage 3 patients (mean eGFR 46.0±20.3 ml/min/1.73m2)39,40. Finally, since this is an observational study we cannot conclude causality without a prospective trial of blood pressure targets in the stage 1–4 CKD population.
In conclusion, in patients with both early and advanced stages of CKD, those with SBP<100 or with higher SBP>120 mm Hg were associated with higher all-cause mortality. The balance of benefits and harms seems to favor more intensive lowering of SBP to 100–120 mm Hg as proposed by the most recent recommendations in the CKD population. The longer-term clinical effects and cost-effectiveness of intensive SBP lowering on all-cause mortality deserves further study.
ACKNOWLEDGEMENTS
The authors dedicate this article to the late Dr. Alexander Goldfarb-Rumyantzev whose bioinformatics expertise, thoughtfulness and insightful ideas made this study possible. His brilliance, humor and humble personality will be sorely missed.
Funding:
MZ is supported by a NIH NIDDK T32 award DK007199.
Footnotes
Conflict of interest statement: None
REFERENCES
- 1.USRDS. 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health. Bethesda, MD; 2020. https://adr.usrds.org/2020. [Google Scholar]
- 2.Tonelli M Chronic Kidney Disease and Mortality Risk: A Systematic Review. J Am Soc Nephrol. 2006;17(7):2034–2047. doi: 10.1681/ASN.2005101085 [DOI] [PubMed] [Google Scholar]
- 3.Gullion CM, Keith DS, Nichols GA, Smith DH. Impact of Comorbidities on Mortality in Managed Care Patients With CKD. Am J Kidney Dis. 2006;48(2):212–220. doi: 10.1053/j.ajkd.2006.04.083 [DOI] [PubMed] [Google Scholar]
- 4.McMahon GM, Preis SR, Hwang S-J, Fox CS. Mid-Adulthood Risk Factor Profiles for CKD. J Am Soc Nephrol. 2014;25(11):2633–2641. doi: 10.1681/ASN.2013070750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung AK, Rahman M, Reboussin DM, et al. Effects of Intensive BP Control in CKD. J Am Soc Nephrol. 2017;28(9):2812–2823. doi: 10.1681/ASN.2017020148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang AR, Loser M, Malhotra R, Appel LJ. Blood Pressure Goals in Patients with CKD: A Review of Evidence and Guidelines. Clin J Am Soc Nephrol. 2019;14(1):161–169. doi: 10.2215/CJN.07440618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021;99(3S):S1–S87. doi: 10.1016/j.kint.2020.11.003 [DOI] [PubMed] [Google Scholar]
- 8.Ku E, Johansen KL, McCulloch CE. Time-Centered Approach to Understanding Risk Factors for the Progression of CKD. Clin J Am Soc Nephrol. 2018;13(5):693–701. doi: 10.2215/CJN.10360917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peralta CA, Shlipak MG, Wassel-Fyr C, et al. Association of antihypertensive therapy and diastolic hypotension in chronic kidney disease. Hypertension. 2007;50(3):474–480. doi: 10.1161/HYPERTENSIONAHA.107.088088 [DOI] [PubMed] [Google Scholar]
- 10.Tomlinson LA, Holt SG, Leslie AR, Rajkumar C. Prevalence of ambulatory hypotension in elderly patients with CKD stages 3 and 4. Nephrol Dial Transplant. 2009;24(12):3751–3755. doi: 10.1093/ndt/gfp357 [DOI] [PubMed] [Google Scholar]
- 11.Johnson ES, Thorp ML, Yang X, Charansonney OL, Smith DH. Predicting Renal Replacement Therapy and Mortality in CKD. Am J Kidney Dis. 2007;50(4):559–565. doi: 10.1053/j.ajkd.2007.07.006 [DOI] [PubMed] [Google Scholar]
- 12.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int Suppl. 2012;2(5):337–414. [Google Scholar]
- 13.Kramer HJ, Townsend RR, Griffin K, et al. KDOQI US Commentary on the 2017 ACC/AHA Hypertension Guideline. Am J Kidney Dis. 2019;73(4):437–458. doi: 10.1053/j.ajkd.2019.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CDC. The National Health and Nutrition Examination Survey. https://www.cdc.gov/nchs/nhanes/index.htm.
- 15.Centers for Disease Control and Prevention. Overview of NHANES Survey Design and Weights. https://www.cdc.gov/Nchs/tutorials/environmental/orientation/sample_design/index.htm. Accessed March 4, 2020.
- 16.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80(1):17–28. doi: 10.1038/ki.2010.483 [DOI] [PubMed] [Google Scholar]
- 18.Brunstrom M, Carlberg B. Association of Blood Pressure Lowering With Mortality and Cardiovascular Disease Across Blood Pressure Levels: A Systematic Review and Meta-analysis. JAMA Intern Med. 2018;178(1):28–36. doi: 10.1001/jamainternmed.2017.6015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McEvoy JW, Daya N, Rahman F, et al. Association of Isolated Diastolic Hypertension as Defined by the 2017 ACC/AHA Blood Pressure Guideline With Incident Cardiovascular Outcomes. JAMA. 2020;323(4):329–338. doi: 10.1001/jama.2019.21402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang TI, Wei G, Boucher R, et al. Baseline Diastolic Blood Pressure and Cardiovascular Outcomes in SPRINT participants with Chronic Kidney Disease. Kidney 360. January 2020:10.34067/KID.0000982019. doi: 10.34067/KID.0000982019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toida T, Sato Y, Ogata S, Wada A, Masakane I, Fujimoto S. Synergic Impact of Body Mass Index, Diabetes, and Age on Long-Term Mortality in Japanese Incident Hemodialysis Patients: A Cohort Study on a Large National Dialysis Registry. J Ren Nutr. December 2019. doi: 10.1053/j.jrn.2019.09.007 [DOI] [PubMed] [Google Scholar]
- 22.Horowitz B, Miskulin D, Zager P. Epidemiology of hypertension in CKD. Adv Chronic Kidney Dis. 2015;22(2):88–95. doi: 10.1053/j.ackd.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Moran AE. Trends in the Prevalence, Awareness, Treatment, and Control of Hypertension Among Young Adults in the United States, 1999 to 2014. Hypertens (Dallas, Tex 1979). 2017;70(4):736–742. doi: 10.1161/HYPERTENSIONAHA.117.09801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303(20):2043–2050. doi: 10.1001/jama.2010.650 [DOI] [PubMed] [Google Scholar]
- 25.Wang F, He K, Wang J, et al. Prevalence and Risk Factors for CKD: A Comparison Between the Adult Populations in China and the United States. Kidney Int Reports. 2018;3(5):1135–1143. doi: 10.1016/j.ekir.2018.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myers OB, Pankratz VS, Norris KC, Vassalotti JA, Unruh ML, Argyropoulos C. Surveillance of CKD epidemiology in the US – a joint analysis of NHANES and KEEP. Sci Rep. 2018;8(1):1–9. doi: 10.1038/s41598-018-34233-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bundy JD, Li C, Stuchlik P, et al. Systolic Blood Pressure Reduction and Risk of Cardiovascular Disease and Mortality: A Systematic Review and Network Meta-analysis. JAMA Cardiol. 2017;2(7):775–781. doi: 10.1001/jamacardio.2017.1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Appel LJ, Wright JT, Greene T. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363(26):2565–2566. doi: 10.1016/j.ycar.2011.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malhotra R, Nguyen HA, Benavente O, et al. Association Between More Intensive vs Less Intensive Blood Pressure Lowering and Risk of Mortality in Chronic Kidney Disease Stages 3 to 5: A Systematic Review and Meta-analysis. JAMA Intern Med. 2017;177(10):1498–1505. doi: 10.1001/jamainternmed.2017.4377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ku E, Bakris G, Johansen KL, et al. Acute Declines in Renal Function during Intensive BP Lowering: Implications for Future ESRD Risk. J Am Soc Nephrol. 2017;28(9):2794–2801. doi: 10.1681/ASN.2017010040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. Blood pressure in CKD.
- 33.Kovesdy CP, Bleyer AJ, Molnar MZ, et al. Blood pressure and mortality in U.S. veterans with chronic kidney disease: a cohort study. Ann Intern Med. 2013;159(4):233–242. doi: 10.7326/0003-4819-159-4-201308200-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boutitie F, Gueyffier F, Pocock S, Fagard R, Boissel JP. J-shaped relationship between blood pressure and mortality in hypertensive patients: new insights from a meta-analysis of individual-patient data. Ann Intern Med. 2002;136(6):438–448. doi: 10.7326/0003-4819-136-6-200203190-00007 [DOI] [PubMed] [Google Scholar]
- 35.Mallamaci F, Tripepi G, D’Arrigo G, et al. Blood Pressure Variability, Mortality, and Cardiovascular Outcomes in CKD Patients. Clin J Am Soc Nephrol. 2019;14(2):233–240. doi: 10.2215/CJN.04030318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang C, Li Y, Zhang J, et al. Prognostic effect of isolated nocturnal hypertension in chinese patients with nondialysis chronic kidney disease. J Am Heart Assoc. 2016;5(10):1–11. doi: 10.1161/JAHA.116.004198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JY, Park JT, Joo YS, et al. Association of Blood Pressure With the Progression of CKD: Findings From KNOW-CKD Study. Am J kidney Dis Off J Natl Kidney Found. January 2021. doi: 10.1053/j.ajkd.2020.12.013 [DOI] [PubMed] [Google Scholar]
- 38.Weiss JW, Peters D, Yang X, et al. Systolic BP and Mortality in Older Adults with CKD. Clin J Am Soc Nephrol. 2015;10(9):1553–1559. doi: 10.2215/CJN.11391114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahman M, Wang X, Bundy JD, et al. Prognostic Significance of Ambulatory BP Monitoring in CKD: A Report from the Chronic Renal Insufficiency Cohort (CRIC) Study. J Am Soc Nephrol. 2020;31(11):2609–2621. doi: 10.1681/asn.2020030236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agarwal R Much to Fear about MUCH. J Am Soc Nephrol. 2020;31(11):2496–2499. doi: 10.1681/ASN.2020091270 [DOI] [PMC free article] [PubMed] [Google Scholar]


