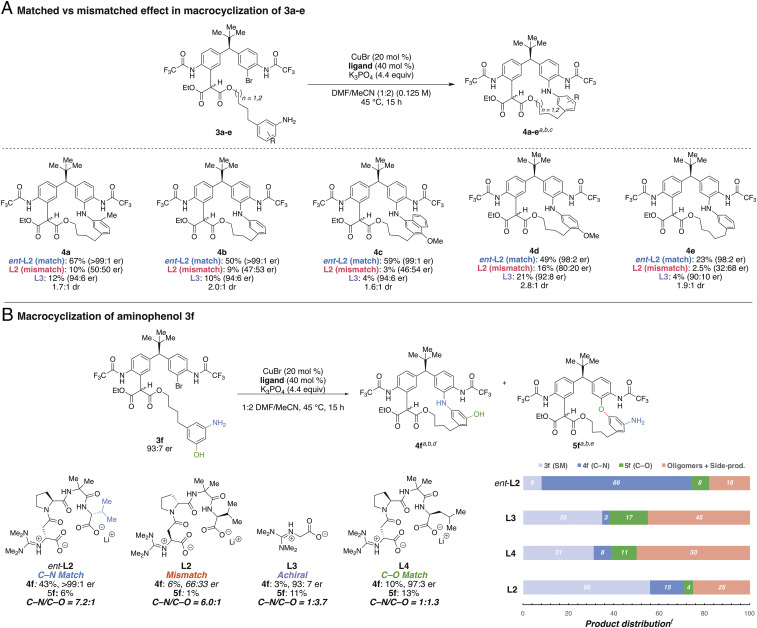
Fig. 4.
(A) Macrocyclization of 3a to 3e: matched and mismatched effect. aIsolated yields. bEnantiomeric ratios were determined using chiral high-performance liquid chromatography analysis. cDiastereomeric ratio (dr) was determined by 1H NMR in CD2Cl2. (B) Macrocyclization of aminophenol 3f using different ligands. d4f is observed as a 2.5:1 mixture of diastereomers at 25 °C in CD2Cl2 by 1H NMR. e5f is observed as a 4.3:1 mixture of diastereomers. fProduct distribution based on uncorrected peak integration on ultraperformance liquid chromatography–mass spectrometry (UPLC-MS) peaks. See SI Appendix, section 14 for UPLC-MS traces.