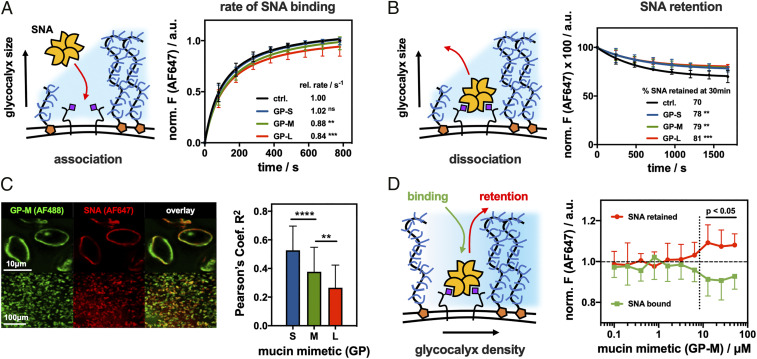
Fig. 3.
Spectator glycocalyx size and density regulate SNA interactions with cell surface receptors. (A) Extended glycocalyx reduces the rate of SNA binding. RBCs were remodeled with S, M, and L mucin mimetics GP (cGP = 7.5 μM), and the binding for AF647-SNA (cSNA = 0.2 μg/mL) was measured by flow cytometry and normalized to untreated cells (n = 6 experimental replicates). (B) Extended glycocalyx reduces SNA dissociation from RBCs. The remodeled cells equilibrated with AF647-SNA were resuspended in pure buffer, and lectin retention was measured by flow cytometry (n = 6 experimental replicates). (C) Extended mucin mimetic glycocalyx drives segregation of SNA–receptor adhesion complexes. Fluorescence micrographs show representative confocal images of RBCs remodeled with mucin mimetic GP-M (green, cGP = 7.5 μM) and stained with AF647-SNA (red, cSNA= 0.2 μg/mL). Bar graph represents Pearson’s correlation coefficient (R2) analysis of images for SNA binding to RBCs remodeled with polymers of all three lengths (cGP = 7.5 μM) (n > 35 individual cell images per polymer condition). (D) Glycocalyx crowding limits SNA association with cell surface receptors while stabilizing the resulting adhesion complex. Association (green) and retention (red) of AF647-SNA at the surface of RBCs remodeled with GP-M at increasing concentration were measured by flow cytometry and normalized to untreated cells (n = 12 experimental replicates). Values represent averages and SDs; P values were determined by ANOVA (A–C) or student test (D); ns>0.05, **<0.01, ***<0.001, ****<0.0001.