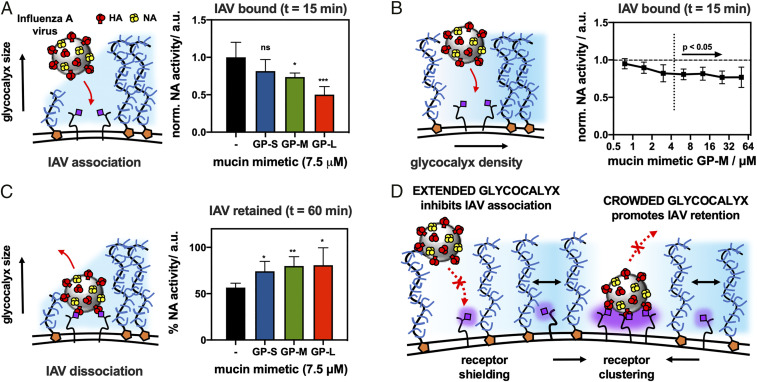
Fig. 5.
Influence of glycocalyx size and density on the binding of H1N1 viruses to sialic acid receptors on RBCs. (A) Extended glycocalyx shields endogenous sialic acid receptors from H1N1 binding. Saturation binding of H1N1 (t = 15 min) to RBCs remodeled with S, M, and L mucin mimetics GP (cGP = 7.5 μM) was assessed via their NA activity toward a fluorogenic substrate 4MU-NANA. (B) Increased glycocalyx crowding limits viral adhesion. Saturation binding of H1N1 (t = 15 min) to RBCs remodeled with increasing concentrations of GP-M (cGP = 0.8 μM to 50.0 μM) was evaluated via NA activity assay with 4MU-NANA. (C) Extended glycocalyx enhances retention of viruses bound to RBC receptors. Retention of H1N1 viruses by RBCs remodeled with mucin mimetics GP of increasing length (cGP = 7.5 μM) was measured as a fraction of NA activity before and after equilibration in fresh buffer (t = 60 min). (D) Proposed model for the influence of a spectator mucin-type glycocalyx on cell−pathogen interactions. Extended and dense glycocalyx shields cell surface receptors from viral adhesion. Glycocalyx crowding drives receptor clustering and enhances retention of bound viruses. Values represent averages and SDs for n = 6 experimental replicates; P values were determined by t test (A and C) and ANOVA (B): *<0.05, **< 0.01, ***<0.001, ****<0.0001.