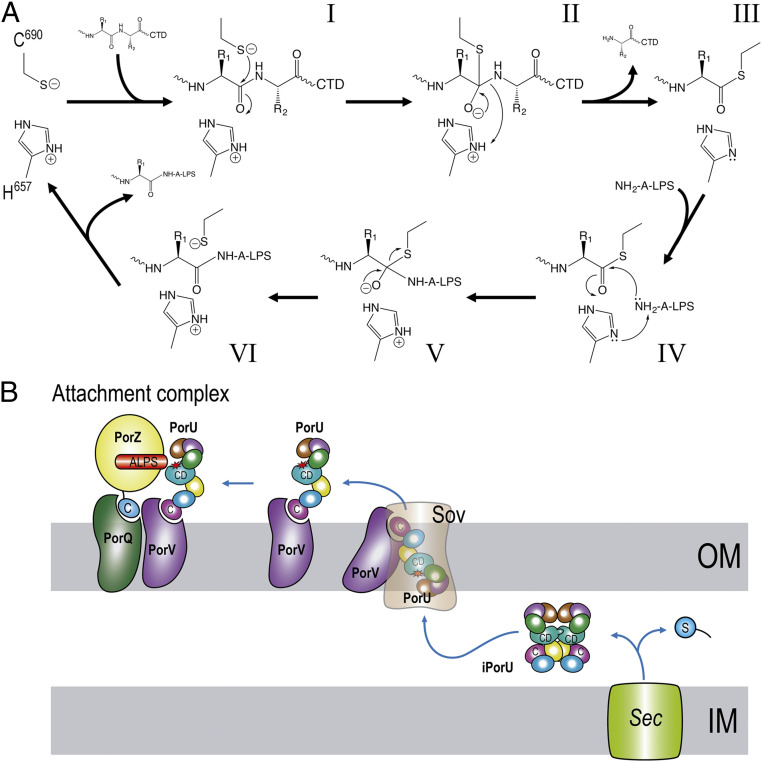
Fig. 6.
Proposed operating modus of PorU. (A) The enzyme likely operates according to a two-step ping-pong mechanism comprising an endopeptidolytic cleavage reaction to remove the CTD (steps I through III) followed by transpeptidation with A-LPS acting as nucleophile (steps IV through VI) for cargoes targeted for insertion into the OM. The nucleophilic group is likely an amine from a phosphoethanolamine group attached to the inner core oligosaccharide of A-LPS (refer also to ref. 25). For secretory cargoes released into the medium, NH2-A-LPS is replaced by other nucleophiles including a water molecule, amines, amino acids, or short peptides. (B) Regulation of PorU activity in the cell involves removal of the signal peptide (blue circle labeled “S”) after Sec translocation to the periplasmic space in which the protein would fold and adopt an inactive dimeric structure (iPorU). Thereafter, conformational rearrangement, hypothetically through interaction with PorV and the Sov translocon, would lead to a monomer (PorU) with a competent CD and a PorV-accessible CTD (labeled “C”) for the transit across the OM. Finally, functional PorU associated with PorV would join PorZ and PorQ in the attachment complex, ready for catalysis, with PorZ presenting A-LPS (25) as one of the two substrates for transpeptidation (refer also to A and Fig. 1). The rest of the T9SS components have been omitted for clarity.