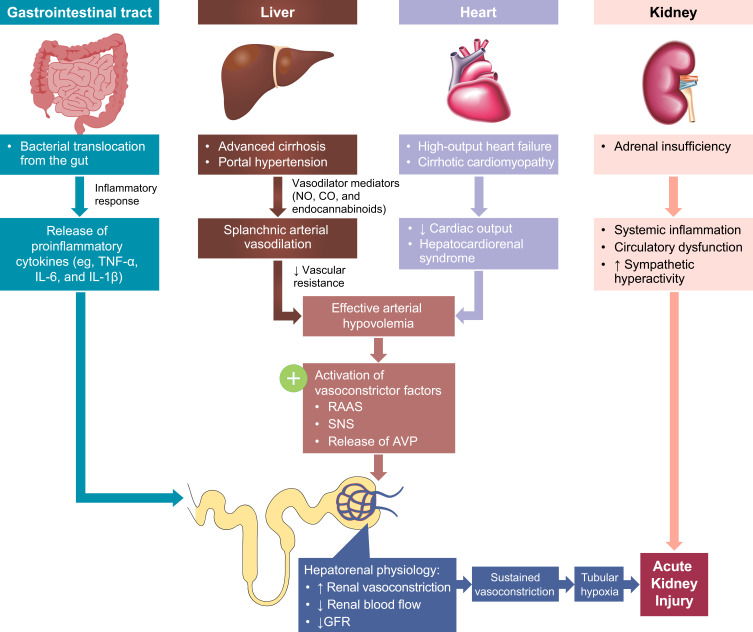
Figure 1.
The multisystemic pathophysiology of HRS-AKI. Renal vasoconstriction is the key pathological mechanism associated with HRS-AKI. Patients with end-stage liver disease develop several multisystemic conditions that contribute to the development of AKI. Portal hypertension secondary to cirrhosis and subsequent vasodilator production leads to splanchnic arterial vasodilation. Additionally, high-output heart failure and cirrhotic cardiomyopathy lead to decreased cardiac output and hepatocardiorenal syndrome. The arterial vasodilation and decreased cardiac output together cause arterial hypovolemia and a reduction in circulatory volume, activating RAAS, SNS, and AVP release to restore circulatory volume. Alternately, in the gut, systemic inflammation secondary to bacterial translocation leads to the release of proinflammatory cytokines. The gastrointestinal and cardiac conditions combined cause increased renal vasoconstriction, decreased renal blood flow, and decreased GFR. This then leads to sustained vasoconstriction, tubular hypoxia, and AKI. Additionally, adrenal insufficiency can also lead to systemic inflammation, circulatory dysfunction, and sympathetic hyperactivity, further exacerbating acute kidney injury.
Abbreviations: AKI, acute kidney injury; AVP, arginine vasopressin; CO, carbon monoxide; GFR, glomerular filtration rate; HRS-AKI, hepatorenal syndrome type of acute kidney injury; IL; interleukin; NO, nitric oxide; RAAS, renin-angiotensin aldosterone system; SNS, sympathetic nervous system; TNF-α, tumor necrosis factor alpha.