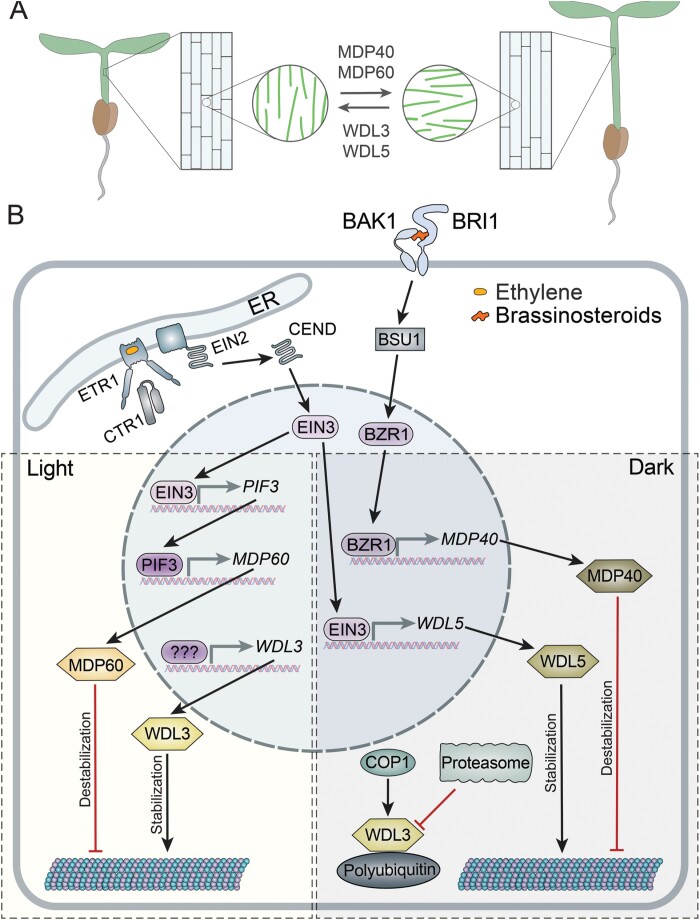
Fig. 5.
Antagonistic interactions between WDLs modulate hypocotyl elongation. (A) WDL3 and WDL5 stabilize microtubules, facilitate longitudinal orientation of microtubules, and reduce cell elongation, whereas MDP40 and MDP60 destabilize microtubules, promote transverse microtubule arrays, and stimulate cell elongation. The balance between activity of these proteins could fine-tune hypocotyl length under either light or dark conditions. (B) Regulation of WDL3, WDL5, MDP40, and MDP60 by light and hormones. Ethylene binding to ETR1 in the endoplasmic reticulum (ER) causes inactivation of kinase CTR1. This results in cleavage of EIN2 C-terminal peptide (CEND). CEND is relocated to the nucleus and activates the transcription factor EIN3. Under light conditions, EIN3 promotes transcription of PIF3; PIF3 promotes transcription of MDP60; MDP60 destabilizes microtubules. WDL3 expression under light promotes microtubule stability. It remains unknown which pathway regulates transcription of WDL3. WDL3 could counteract MDP60. In the dark, the cytoplasmic COP1 ubiquitinates WDL3 and triggers its degradation. In parallel, EIN3 promotes transcription of WDL5, and WDL5 stabilizes microtubules. Brassinosteroids bind the BRI1–BAK1 receptor complex that, through several cytoplasmic proteins, including BSU1, activates BZR1. BZR1 promotes transcription of MDP40. Destabilization of microtubules by MDP40 could counteract WDL5. Activating interactions are shown in red and inhibiting interactions are shown in red.