FIGURE 1.
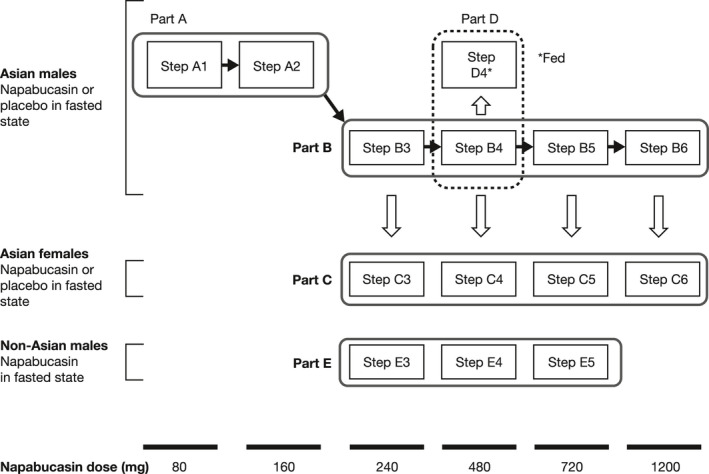
Study design. Part A assessed safety, tolerability, and pharmacokinetics of a single dose of napabucasin or placebo, in Asian males in the fasted state. Parts B and C assessed QT/QTc interval prolongation, safety, tolerability, and pharmacokinetics of a single dose of napabucasin or placebo in Asian males (Steps B3 to B6) and Asian females (Steps C3 to C6) in the fasted state. Part D assessed safety, tolerability, and pharmacokinetics of a single dose of napabucasin in Asian males from Step B4 following a typical Japanese breakfast consumed over 20 min. Part E assessed safety, tolerability, and pharmacokinetics of a single dose of napabucasin in non‐Asian males in the fasted state
