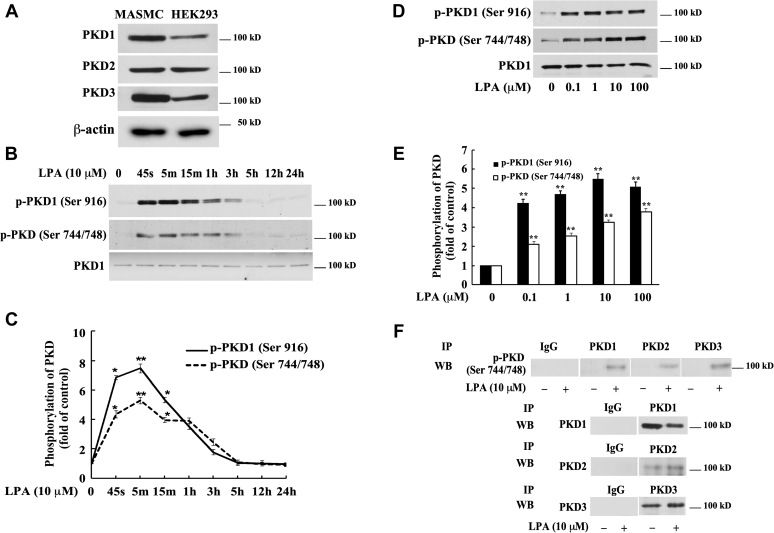
Figure 1.
LPA induces activation of protein kinase D (PKD) in mouse aortic smooth muscle cells (MASMCs).A, Western blot analysis of the expression of PKD isoforms in MASMCs using antibodies against PKD1, PKD2, and PKD3. Lysates of HEK293 cells were used as a positive control. β-actin serves as a loading control. B, Western blot analysis of the time course of LPA stimulation of PKD phosphorylation in MASMCs. Antibodies against p-PKD1 (Ser916) and p-PKD activation loop (Ser 744/748) were used. PKD1 protein expression is shown in the bottom panel. C: time-dependent PKD phosphorylation was quantified by densitometry. Data are mean ± SD from three experiments. D, Western blot analysis of the concentration dependence of LPA induction of PKD phosphorylation. MASMCs were stimulated with LPA for 5 min. E, concentration-dependent PKD phosphorylation induced by LPA was quantified by densitometry. Data are mean ± SD from three experiments. ∗p < 0.05 and ∗∗p < 0.01 versus control. F, immunoprecipitation results of phospho-PKD three isoforms (PKD1–3). Harvested protein samples were immunoprecipitated with specific PKD1, PKD2, and PKD3 antibodies followed by Western blotting analysis for detection of each of the three PKD activations (first panel) with antibody against p-PKD activation loop (Ser744/748). The lower panels (2–4) indicate the efficiency of the immunoprecipitation with each of the specific PKD antibodies. IgG immunoprecipitation serves as a negative control.