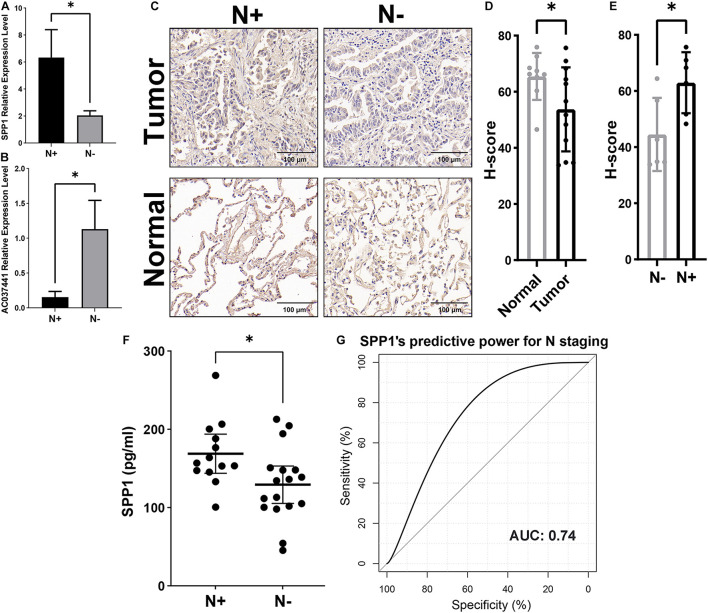
FIGURE 8.
Validation of SPP1 as a potential biomarker for early lymph node metastasis by quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR), immunohistochemical (IHC), and enzyme-linked immunosorbent assay (ELISA) assays. The expression levels of SPP1 (A) and lncRNA (AC037441) (B) in tumor samples were compared by qRT-PCR in the lymph node metastasis group versus the non-metastatic group. (C) The representative IHC figures of SPP1 in Stage-T1 LUAD patients with and without lymph node metastasis. (D) The H-scores of SPP1 in tumor tissues (n = 12) were lower than that of adjacent normal tissues (n = 9). (E) The tumor sample scores distinction of SPP1 in the lymph node metastatic versus non-metastatic groups. (F) Comparison of the SPP1 concentration in the plasma of Stage-T1 LUAD patients between patients with lymph node metastasis and those without metastasis. (G) The receiver operating curve (ROC) and area under the curve (AUC) were used to evaluate the predictive efficiency of plasma SPP1 concentrations for early lymph node metastasis. *p < 0.05.