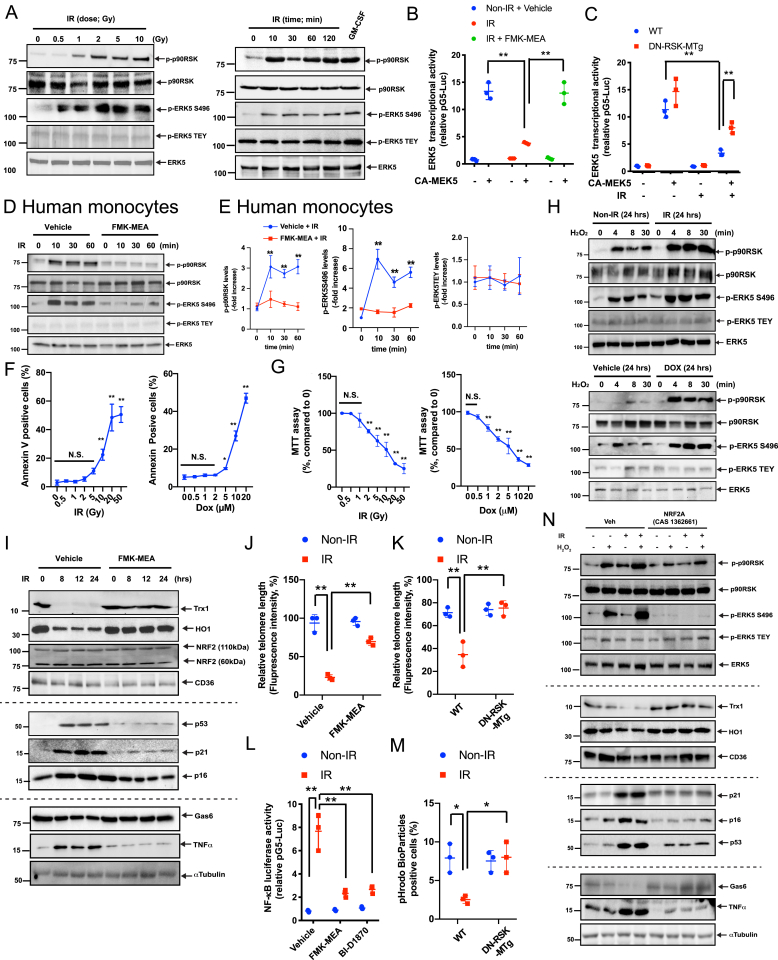
Fig. 1.
IR and DOX sensitized macrophages to oxidative stress and induced SASP, which are inhibited by inhibiting p90RSK activity and activating NRF2.
(A) BMDMs were analyzed by immunoblotting at various time points after IR (2 Gy) or with varying doses for 10 min. Western blot analyses were performed using specific antibodies indicated on the right. GM-CSF stimulation (20 ng/ml for 30 min) was used as the positive control. Representative images from 3 independent experiments are shown (quantification in Figs. S1A and B). (B, C) BMDMs were transfected with pBind-ERK5 and pG5-luc plasmids and pcDNA3.1 plasmid or CA-MEK5 for 30 h. They were then pre-treated with FMK-MEA (10 μM) or vehicle (DMSO, 0.1%) for 1 h and exposed to IR (2 Gy) (B). Alternatively, BMDMs from WT and DN-p90rsk-MTg mice were treated with IR, and after 6 h, ERK5 transcriptional activity was measured as described in Methods (C). Mean ± SD (n = 3). (D) Human monocytes were pre-treated with FMK-MEA (10 μM) or vehicle for 30 min and then exposed to IR (2 Gy). Cells were collected at the indicated times after IR, and total p90RSK, p90RSK phosphorylation, ERK5 S496 phosphorylation, ERK5 TEY motif phosphorylation, and total ERK5 were detected by Western blotting. (E) Quantification of IR-induced p90RSK S380 phosphorylation (left), ERK5 S496 phosphorylation (middle), and ERK5 TEY motif phosphorylation (right) is shown after normalization by total protein levels. The data represent the mean ± SD (n = 3). Blue line: DMSO control pre-treatment, red line: FMK-MEA pre-treatment. (F) Percentage of apoptotic BMDMs after 24 h treatment with IR or DOX is shown at the indicated doses. Cells were stained with annexin V and analyzed by flow cytometry. Data are expressed as mean ± SD (n = 3) from at least 3 independent experiments. G) MTT assays were performed to measure cell viability after 24 h of IR and DOX treatment at the indicated doses. Results are expressed as % compared to time 0. Mean ± SD, (n = 3). (H) BMDMs were exposed to IR (2 Gy), DOX (1 μM), or vehicle and after 24 h, cells were incubated with H2O2 (200 μM) for 0–30 min. Western blotting was performed with the indicated antibodies. Representative images from 3 independent experiments are shown. (I) BMDMs were pre-treated with FMK-MEA (10 μM) or vehicle for 1 h and irradiated by IR. After 0–24 h, Western blotting was performed with the indicated antibodies. Representative images from 3 independent experiments are shown. (J, K) BMDMs were pre-treated with FMK-MEA (10 μM) or vehicle for 1 h and then exposed to IR. (J), Alternatively, BMDMs from WT and DN-p90rsk-MTg mice were exposed to IR. After 24 h, TL lengths were determined by measuring the fluorescent telomeric signal intensity of the fluorescein-conjugated PNA probe as described in Methods. Results are presented as the relative TL length (%) (mean ± SD). The TL length of the human T-cell leukemia cell line (1301) cells was set to 100% (n = 3). (L) BMDMs were transfected with the NF-κB luciferase reporter and the constitutively expressing Renilla luciferase vector for 16 h. Cells were pre-treated with FMK-MEA (10 μM) or BI-D1870 (5 μM) for 1 h and then exposed to IR or left untreated. After 12 h, NF-κB transcriptional activity was measured as described in Methods. Mean ± SD, (n = 3). (M) BMDMs were isolated from WT and DN-p90rsk-MTg mice and exposed to IR or left untreated. After 24 h, cells were incubated with the IncuCyte pHrodo-labeled apoptosis detection probe, and pHrodo-positive cells quantified. Mean ± SD, (n = 3). (N) BMDMs were pre-treated with NRF2A (CAS 1362661) or vehicle for 6 h and exposed to IR or non-IR. After 18 h, BMDMs were incubated with H2O2 (200 μM) for 10 min. Western blotting was performed using specific antibodies indicated on the right. Representative images from 3 independent experiments are shown. **P < 0.01.