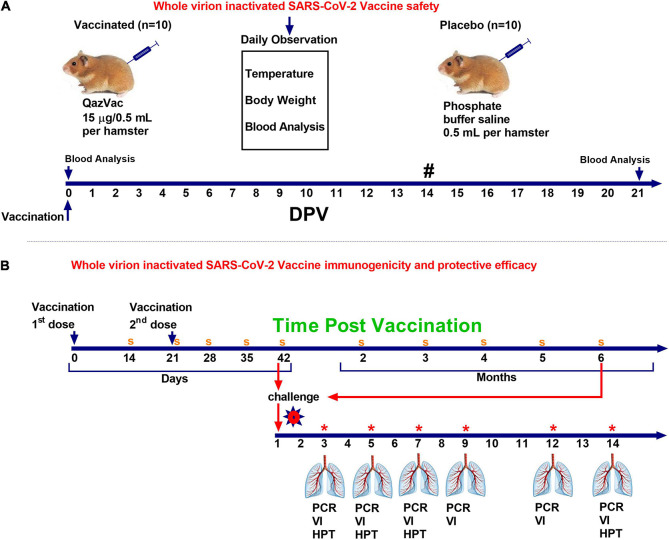
FIGURE 2.
Study design of QazCovid-in vaccination in Syrian hamsters. (A) The study of the safety of the inactivated QazCovid-in vaccine in Syrian hamsters. (B) The determination of immunogenicity and evaluation of the protective efficacy of the inactivated QazCovid-in vaccine in Syrian hamsters. PCR, polymerase chain reaction; VI, virus isolation; HPT, histopathology. Notes (#)—measurement of body temperature and body weight was carried out within 14 days. (s)—sampling of blood serum to determine the titer of viurs-neutralizing antibodies (*)—on 3, 5, 7, 9, 12, and 14 DPI, samples of nasal and oral swabs were collected and examined by PCR and viral isolation in cell culture.