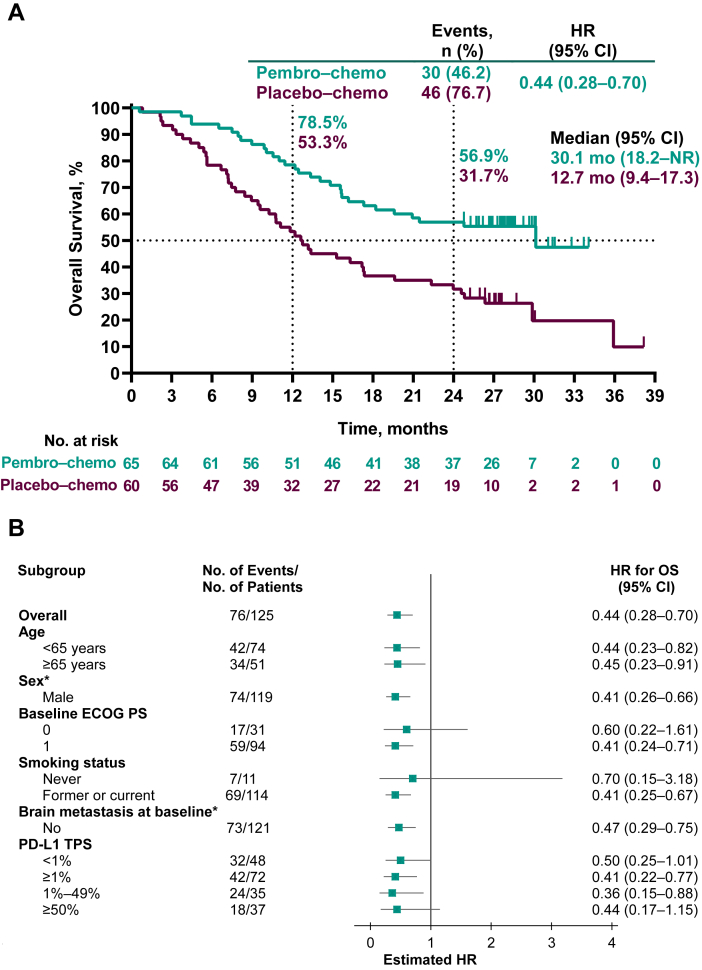
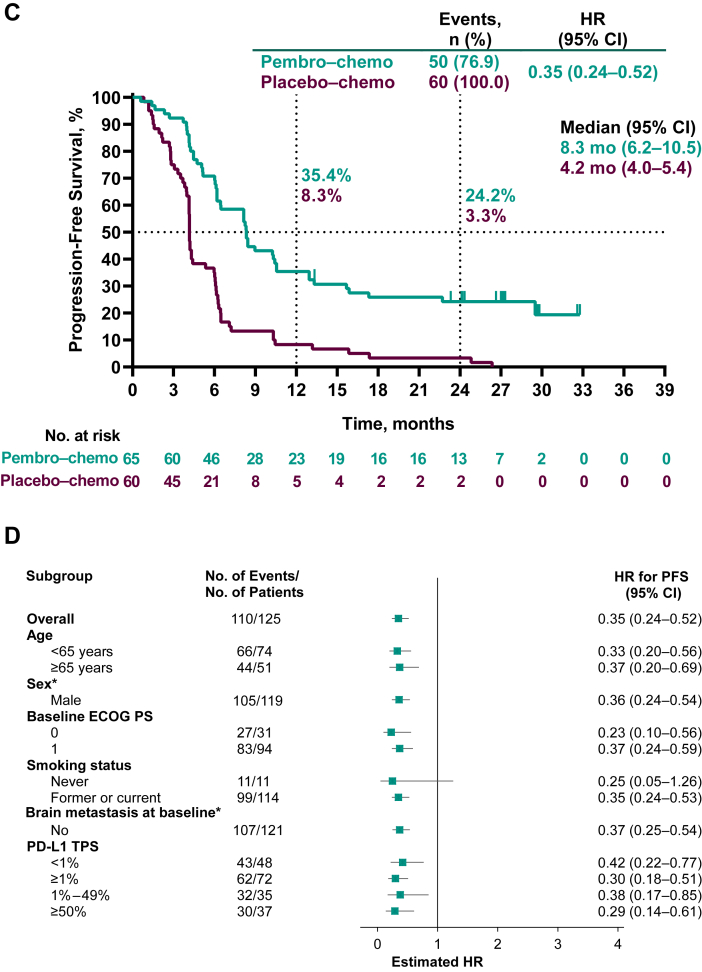
Figure 2.
Kaplan-Meier analysis of overall survival (A) in the intention-to-treat population and (B) in key patient subgroups and progression-free survival assessed by BICR per RECIST version 1.1 (C) in the intention-to-treat population and (D) in key patient subgroups. Patients with tumors who were not assessable for PD-L1 TPS were not included in the relevant subgroup analyses. ∗Data not presented for subgroups of “Female” (n = 6) and for patients with brain metastasis at baseline (n = 4) owing to very few patients which precludes any meaningful analysis. BICR, blinded independent central review; Chemo, chemotherapy; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; pembro, pembrolizumab; NR, not reached; OS, overall survival; PD-L1, programmed death-ligand 1; RECIST, Response Evaluation Criteria in Solid Tumors; TPS, tumor proportion score.