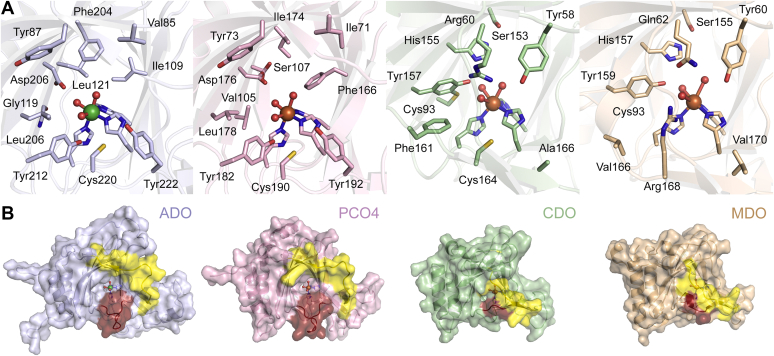
Figure 5.
Comparisons of active site architecture and cavity opening of human ADO with other thiol dioxygenases.A, active site architecture and B, surface representations of hADO, PCO4, hCDO, and MDO (from left to right). The active site residues of hADO are highly conserved in PCO4 but distinct from hCDO and MDO. In ADO and PCO, regions colored in yellow and red indicate the border region and the hairpin loop, respectively. However, in CDO and MDO, the corresponding yellow regions function as a “lid” of the catalytic cavity, and the corresponding hairpin loop regions are absent. Residues of the yellow regions are 249–261 in hADO, 220–231 in PCO4, 174–182 in hCDO, and 177–185 in MDO. Residues of red regions are 212–220 in hADO, 182–190 in PCO4, 161–164 in hCDO, and 163–168 in MDO. PDB entries 7REI, 6S7E, 6E87, and 4TLF (from left to right). The orientation is identical to the left panel of Figure 3A.