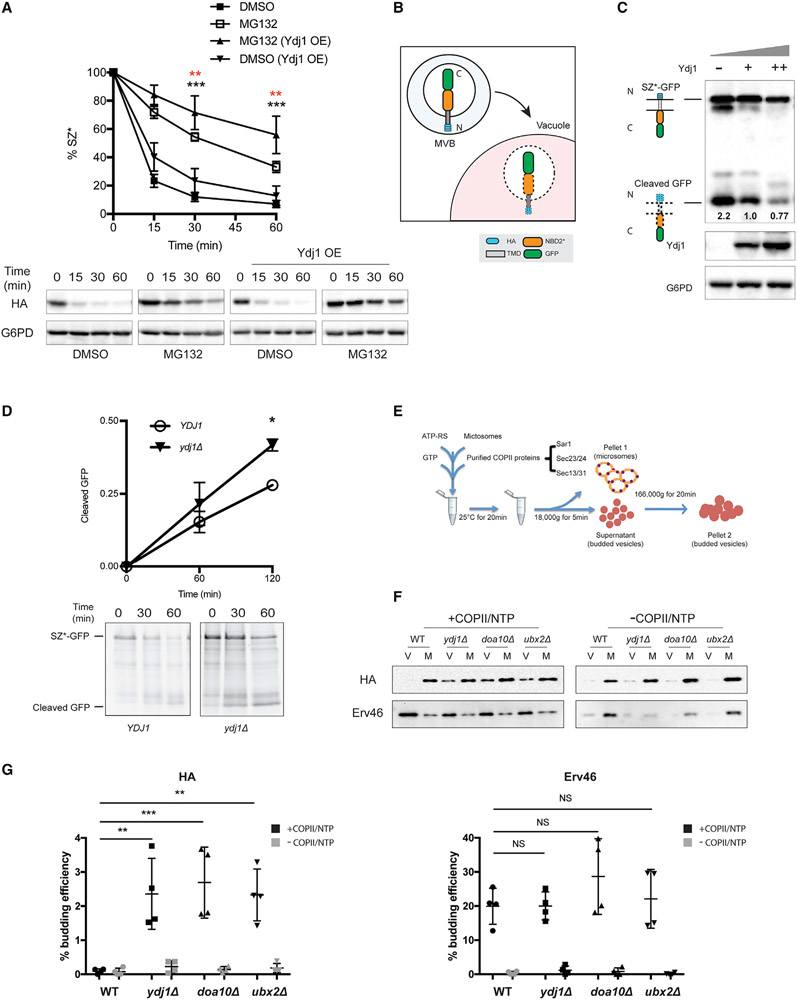
Figure 2. Ydj1 facilitates the ER retention of SZ*.
(A) Proteasome-dependent degradation of SZ* was measured in pdr5Δ yeast expressing both SZ* and Ydj1 versus a vector control in the presence or absence of 100 μM MG132. Data are the means ± SE of 6–9 independent experiments; black asterisks apply to the MG132 (Ydj1 OE) versus DMSO (Ydj1 OE) comparison, and red asterisks apply to the MG132 (Ydj1 OE) versus MG132 comparison. **p < 0.005, ***p < 0.0005.
(B) A schematic of SZ*-GFP sorting into the vacuole by the MVB pathway. N and C indicate SZ*-GFP topology with the N terminus facing the ER lumen (not shown), but the protein is then delivered to the vacuole in MVBs.
(C) GFP cleavage in yeast expressing variable amounts of Ydj1 was determined by immunoblotting. –, denotes ydj1Δ; +, denotes YDJ1 (wild-type); ++, denotes wild-type yeast overexpressing Ydj1. Numbers indicate average values of normalized GFP cleavage levels from 3 independent experiments.
(D) GFP cleavage was measured by pulse-chase in wild-type and ydj1Δ yeast. Cleavage was determined by normalizing free GFP to full-length SZ*-GFP at 0 min. Data are the means ± SE of 3 independent experiments; *p < 0.05.
(E) A schematic of the in vitro budding assay to measure ER exit by COPII vesicles.
(F) In vitro COPII budding efficiency of SZ*-GFP in the presence or absence of Ydj1, Doa10, or Ubx2, respectively, was measured. V, 50% of total budded vesicles; M, 2.5% unbudded microsomes used in the reaction; +COPII/NTP and −COPII/NTP, indicate experiments performed in the presence or absence of the purified COPII proteins and energy, respectively.
(G) A graph depicting in vitro budding efficiency of SZ*-GFP and the positive control Erv46, as quantified from part (F). Data are the means ± SE of 4 independent experiments; **p < 0.005; NS, not significant.