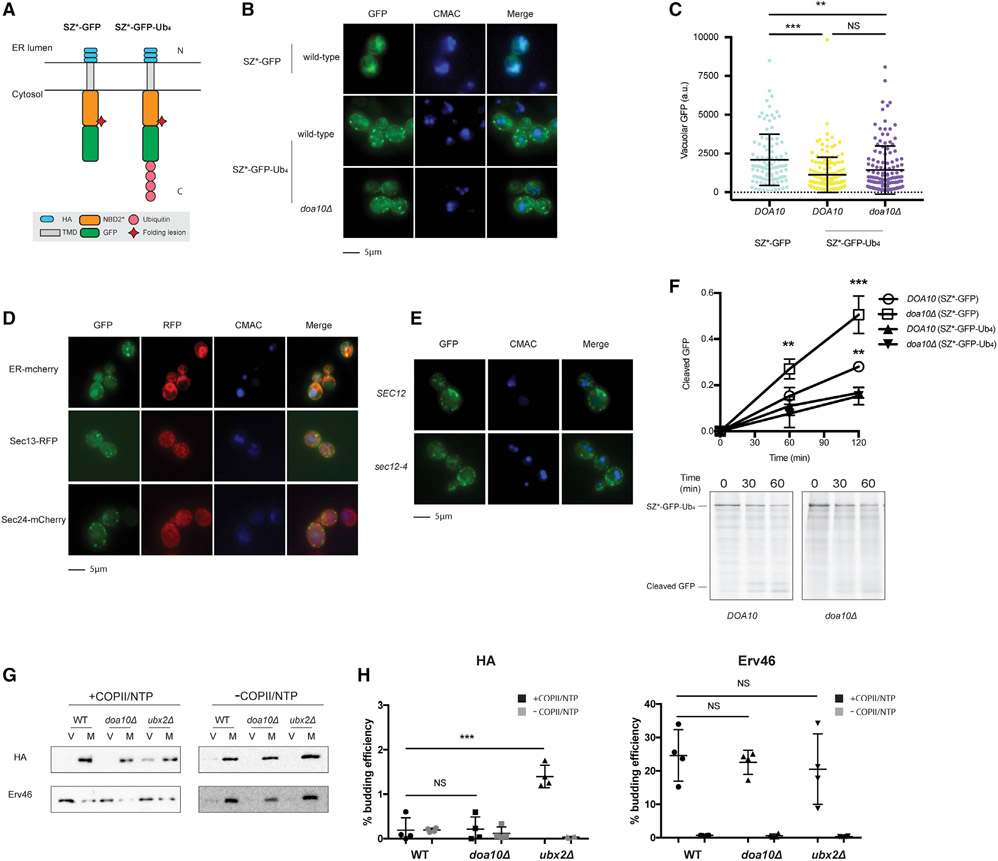
Figure 4. Tetra-ubiquitin attenuates ER exit.
(A) Predicted topologies of SZ*-GFP and SZ*-Ub4. N and C indicate the topology with respect to the ER.
(B) Live-cell fluorescence and DIC imaging of SZ*-Ub4 in wild-type and doa10Δ yeast and SZ*-GFP in wild-type yeast. CMAC marks the yeast vacuole.
(C) Quantification of GFP intensity in the vacuole from (B). Greater than 100 cells in each strain were counted; **p < 0.005, ***p < 0.0005.
(D) Live-cell fluorescence imaging of wild-type yeast expressing the integrated fluorescent marker mCherry-Scs2-tm (ER-cherry), Sec13-RFP, or Sec24-mCherry, and containing the SZ*-Ub4 expression plasmid.
(E) Live-cell fluorescence imaging of wild-type or sec12-4 yeast expressing SZ*-Ub4. Cultures were shifted to 37°C for 30 min prior to imaging.
(F) GFP cleavage was measured by pulse-chase in wild-type and doa10Δ yeast. Cleavage was determined by normalizing free GFP to full-length SZ*-Ub4 at 0 min. Data are the means ± SE of 3 independent experiments; *p < 0.05. Quantification of SZ*-GFP in wild-type and doa10Δ yeast is also shown.
(G) In vitro COPII budding efficiency of SZ*-Ub4 in the presence or absence of Doa10 or Ubx2, respectively, was measured. V, 50% of total budded vesicles; M, 2.5% unbudded microsomes used in the reaction; +COPII/NTP and −COPII/NTP, indicate experiments performed in the presence or absence of the purified COPII proteins and energy, respectively.
(H) Graph depicts in vitro budding efficiency of SZ*-GFP as quantified from (G). Data are the means ± SE of 4 independent experiments; ***p < 0.0005.