Fig. 1. Plasma neutralizing activity.
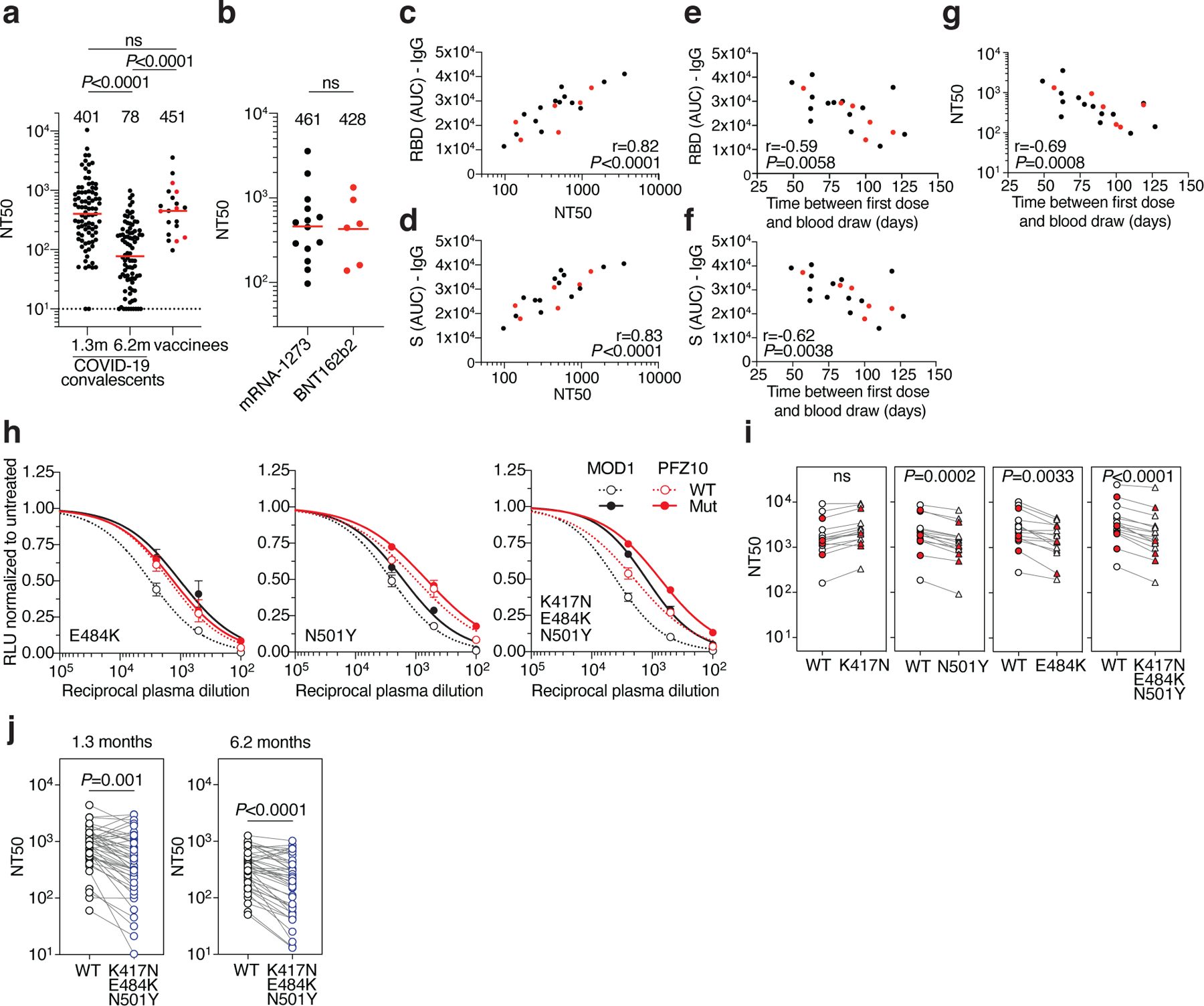
a, SARS-CoV-2 pseudovirus neutralization assay. NT50 values for COVID-19 convalescent plasma measured at 1.3 months8 and 6.2 months7 after infection as well as plasma from vaccinees. NT50 values lower than 10 were plotted at 10. Mean of 2 independent experiments. Red bars and indicated values represent geometric mean NT50 values. Statistical significance was determined using the two-tailed Mann-Whitney U-test. Pre-COVID-19 historical control plasma was analyzed as a negative control and showed no detectable neutralization (NT50<10). b, NT50 values for Moderna mRNA-1273 (black) and Pfizer-BioNTech BNT162b2 (red) vaccine recipients. Red bars and indicated values represent geometric mean NT50 values. Statistical significance was determined using the two-tailed Mann-Whitney U-test. c, Anti-RBD IgG AUC (Y axis) plotted against NT50 (X axis) r=0.82, p<0.0001. d, Anti-S IgG AUC (Y axis) plotted against NT50 (X axis) r=0.83, p<0.0001. e, Anti-RBD IgG AUC (Y axis) plotted against time between first dose and blood draw (X axis) r=−0.59 p=0.0058. f, Anti-S IgG AUC (Y axis) plotted against time between first dose and blood draw (X axis) r=−0.62 p=0.0038. g, NT50 (Y axis) plotted against time between first dose and blood draw (X axis) r=−0.69 p=0.0008. The r and p values for correlations in c-g were determined by two-tailed Spearman correlation. Moderna vaccinees are in black and Pfizer-BioNTech in red. h. Examples of neutralization assays, comparing the sensitivity of pseudotyped viruses with WT and RBD mutant SARS-CoV-2 S proteins to vaccinee plasma. MOD1 and PFZ10 indicate two representative individuals receiving the Moderna and Pfizer-BioNTech vaccine, respectively (for details see Ext. Data Table 1). i, NT50 values for vaccinee plasma (n=15) neutralization of pseudotyped viruses with WT and the indicated RBD-mutant SARS-CoV-2 S proteins. Pfizer-BioNTech vaccinees in red. j, NT50 values for convalescent plasma (n=45) neutralization of pseudotyped viruses with WT and KEN (K417N/E484K/N501Y) SARS-CoV-2 S proteins. Statistical significance in i and j was determined using one tailed t-test. All experiments were performed a minimum of 2 times. Pseutotyped viruses containing the E484K mutation and corresponding WT controls contain the R683G mutation (for details see methods).
