Extended Data Fig. 7: Neutralizing activity of monoclonal antibodies in clinical development against SARS-CoV-2 variants.
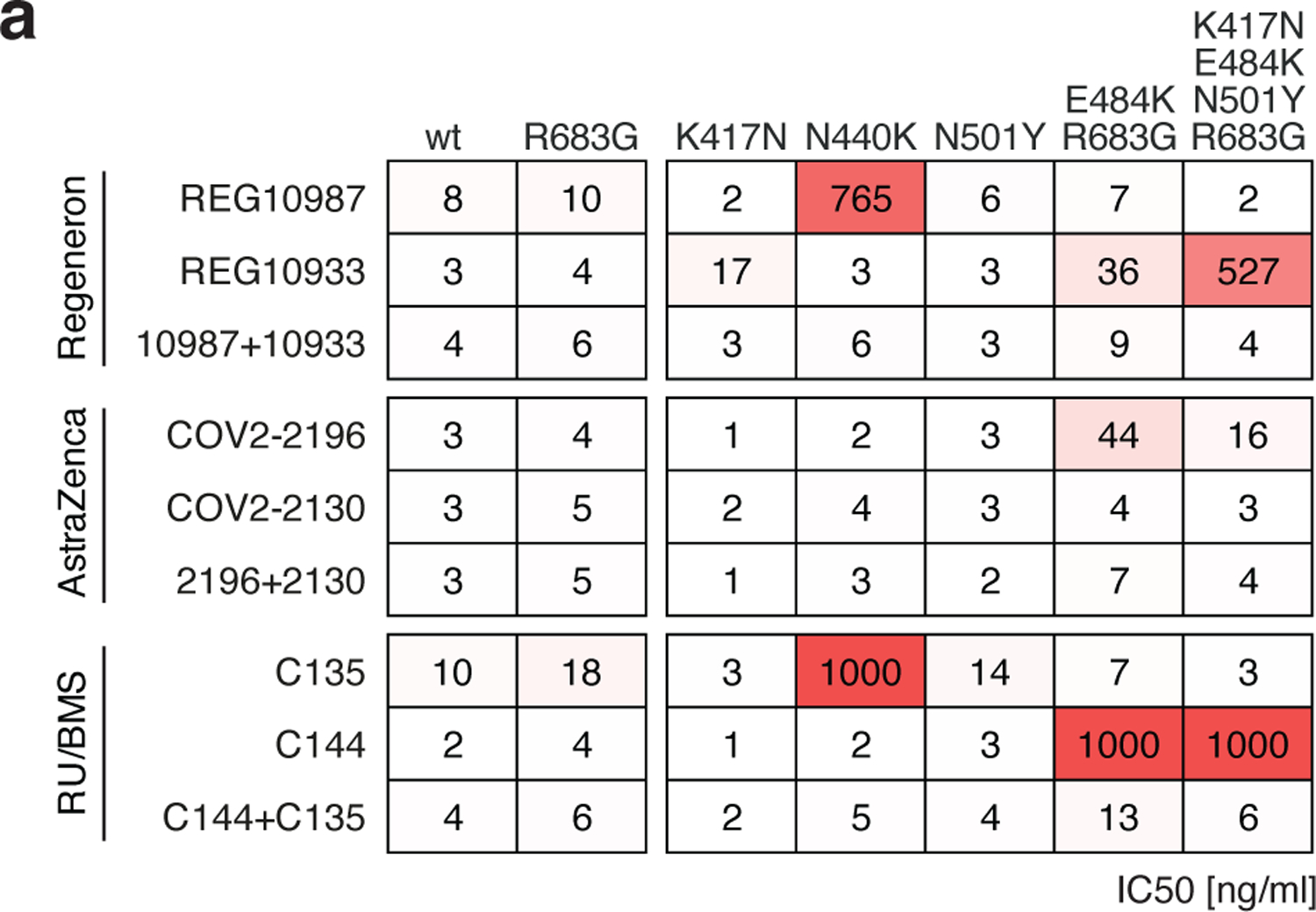
a, Results of a SARS-CoV-2 pseudovirus neutralization assay. IC50 values for 6 different monoclonal antibodies, alone or in their clinically designated combinations, for neutralization of wild type and the indicated mutant SARS-CoV-2 pseudotyped viruses. Antibodies with IC50 values above 1000 ng/ml were plotted at 1000 ng/ml. Data are the mean of 2 independent experiments. Color gradient indicates IC50 values ranging from 0 (white) to 1000 ng/ml (red). The combination of REGN 10987 and 10933 (casirivimab and imdevimab, respectively)13,66,67 has been granted emergency use authorization by the U.S. FDA, the combination of COV2–2196 and COV2–2130 (licensed to Astra Zeneca as AZD7442)26, and the combination of C135 and C144 (The Rockefeller University)8 are currently in clinical trials (NCT04507256 and NCT04700163, respectively).
