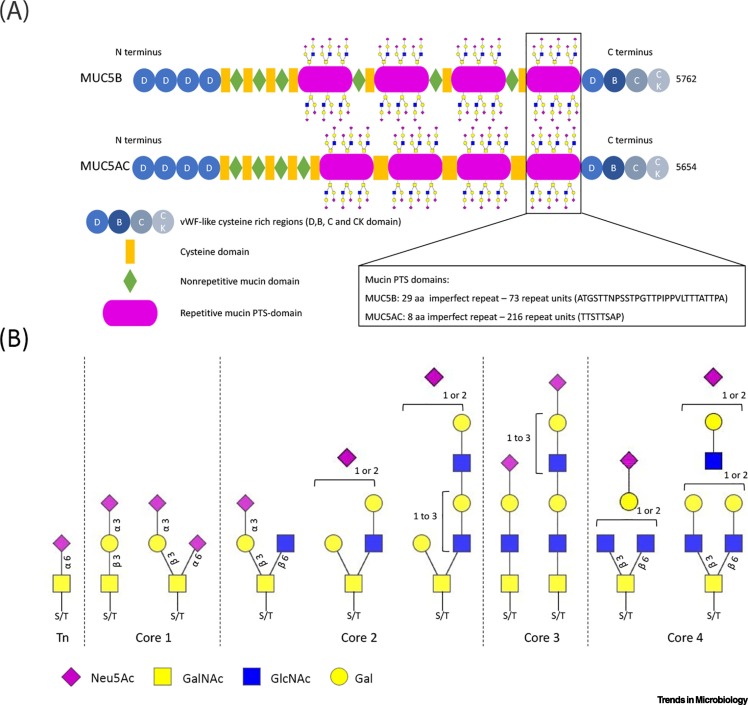
Figure 2.
Mucins and their glycosylation.
(A) The domain structure of the soluble mucins MUC5B (5762 amino acids) and MUC5AC (5654 amino acids). The N- and C-terminal von Willebrand factor (vWF)-like regions and cysteine-rich domains are highly conserved between MUC5B and MUC5AC as well as between species. The four central proline/threonine/serine-rich (PTS) regions consist of imperfect repeats (aa; amino acids). PTS repeats are densely decorated with O-linked glycans and their low sequence conservation between species will result in spatial differences in glycan presentation that could potentially affect the binding of a specific virus. (B) Diversity of sialylated O-linked glycan structures present on high-molecular-weight human mucins. Structures were interpreted from the glycan compositions reported in the most extensive analysis of glycans on mucus to date [39]. Note that di-sialylated bi-antennary structures with multiple LacNAc repeats are present. Such structures on mucins are likely to have differential effects on the binding of viruses as has been reported for N-linked glycans [77]. Sulfation of the sialoglycans shown here was also abundant [39] but is not indicated. Abbreviations: Gal, galactose; GalNAc, N-acetylgalactosamine; GlcNAc, N-acetylglucosamine; Neu5Ac, N-acetyl-neuraminic acid.