Abstract
Objectives
Nutrition has become an important component in treating individuals during the coronavirus disease of 2019 (COVID-19) pandemic, which is increasingly affecting the world population and causing a collapse in health services. Prolonged hospitalization, including immobilization and catabolism, induces a decrease in body weight and muscle mass that may result in sarcopenia, a condition that impairs respiratory and cardiac function and worsens the prognosis. The present study aimed to analyze enteral nutritional support and the clinical evolution of patients admitted with COVID-19 in Brazil.
Methods
This was a retrospective study, conducted from March to May 2020, of patients admitted to a referral hospital in cardiology and pulmonology in Fortaleza-Ce/Brazil. Two hundred patients infected with COVID-19 were selected for the study. Sociodemographic, clinical, and nutritional data were collected from electronic medical records, and associations between outcomes and the use of the prone body position with nutritional variables were analyzed by linear regression. Odds ratio and 95% confidence interval estimates for the death outcome were analyzed by logistic regression.
Results
Of the 112 patients who were fed by enterally, the majority were male (n = 61; 54.5%), elderly (n = 88; 78.6%), and with no current smoking habit (n = 81; 72.3%). The median hospital stay was 14 d, mostly in intensive care units (median: 9 d). Prone body positioning impacted the nutritional therapy. In general, patients who maintained a prone body position tested lower for kcal/kg of body weight, protein/kg of body weight, percentage of diet adequacy, and total caloric value. In addition, patients who died had a lower mean maximum kcal/kg body weight, protein/kg body weight, percentage of diet adequacy, and total caloric value compared with surviving patients.
Conclusions
An association between inadequacies in protein and energy supply with mortality was confirmed, suggesting that nutritional support optimization should be prescribed in such situations.
Keywords: Coronavirus infections, Enteral nutrition, patients
Introduction
Human nutrition plays a fundamental role in protecting against infectious diseases and has a direct relationship with our immune system. Individuals with nutritional deficiencies present with reduced blood cell production, resulting in impaired defense cells and increased risk of infections [1,2]. Nutritional support is extremely important to fight against and prevent infections, especially in individuals affected by the new human coronavirus [3].
A new coronavirus appeared in December 2019 in the Chinese city Wuhan, a virus that was until then of unknown origin [4]. Almost 2 mo after registration of the first case, the new virus was recognized as coronavirus type 2, which is associated with severe acute respiratory syndrome (SARS) and responsible for the coronavirus disease of 2019 (COVID-19). Thus, what was a local epidemic has turned into a global pandemic, and the consequences remain uncertain and tragic according to the World Health Organization [5]. By May 18, 2020, a total of 4 618 821 cases of COVID-19 had been confirmed worldwide with 311 847 deaths [6]. On May 19, 2020, Brazil reported 271 628 confirmed cases and 17 971 deaths [7]. The data represent the peak of the first wave in the year 2020, and these figures alone already define the disease, its degree of dissemination, and its lethality.
Prolonged hospitalization, including immobilization and catabolism, induces a decrease in body weight and muscle mass that can result in sarcopenia, which is a condition that impairs respiratory and cardiac functions and worsens prognoses. In this scenario, there is an urgent need for nutritional guidance to prevent or oppose hospital malnutrition and improve a patient's response to therapy [8].
COVID-19 has demonstrated the potential to damage the respiratory system in affected individuals. In addition, as evidenced in the demographic data, secondary conditions (i.e., smoking, cardiometabolic and pulmonary diseases) are associated with complications and susceptibilities involving respiratory muscle performance. In addition, obese patients admitted to intensive care and treated mechanical ventilation have been shown to have a higher risk of severe acute respiratory syndrome [9].
The Brazilian Society of Nutritional Therapy (BRASPEN) published a document reviewing nutritional assistance for patients hospitalized with COVID-19. The national publication corroborates global guidelines [10,11], emphasizing that all patients benefit from early and individualized nutritional therapy. Oral supplementation and/or enteral nutritional therapy (ENT) prescriptions are recommended for patients who are unable to orally reach 60% of their energy needs [12]. Even patients infected with COVID-19 who are not at risk of malnutrition should maintain adequate protein (1.5 g/kg/d) and caloric (25–30 kcal/kg/d) intake, and vitamins and micronutrients can potentially benefit infected patients due to their antiinflammatory and antioxidant properties [13].
Considering national and international recommendations, patient monitoring is necessary to reduce nutritional deficits, avoid intense catabolism, and shorten mechanical ventilation regimens. Nutrition is important to cope with this pandemic, because nutrition generates a better immune response, reduces infectious complications, and results in a better prognosis for the patient. The present study aimed to analyze adequacy of enteral nutritional support and the clinical evolution of patients admitted for COVID-19 in a referral hospital in cardiology and pulmonology in Brazil.
Methods
Study design
This retrospective study was conducted from June to December of 2020 with data from patients hospitalized at intensive care units (ICUs) specializing in COVID-19 from March to May 2020 at a referral hospital in cardiology and pulmonology in Fortaleza-Ce/Brazil. The hospitalization of patients occurred at the time of disease peak and the collapse of the city hospital system [14]. The hospital has a capacity of 262 beds for COVID-19 treatment, of which 179 were for wards and 83 for intensive care. For this study, a total of 200 patients were evaluated. Ethical research precepts with human beings based on Resolution No. 466/2012 of the National Health Council were respected. The study was approved by the ethics committee of the hospital and the University of Fortaleza (opinion 4.088.500/2020).
Population and sample
The target population of the study was composed of patients hospitalized at the ICU of the COVID-19 ward of a health institution. The sample consisted of adult men and women, age >19 y, with a positive diagnosis of COVID-19 in real-time reverse-transcription polymerase chain reaction or quick tests. The sample was selected according to the date of hospital admission during the study period. All patients with records of prescriptions and evolution of enteral diets in their medical records were included. Retrospective data collection was performed by analyzing the records from the hospital nutrition service and the electronic medical records. All patients who participated in the study had received a medical diet prescription (oral or enteral).
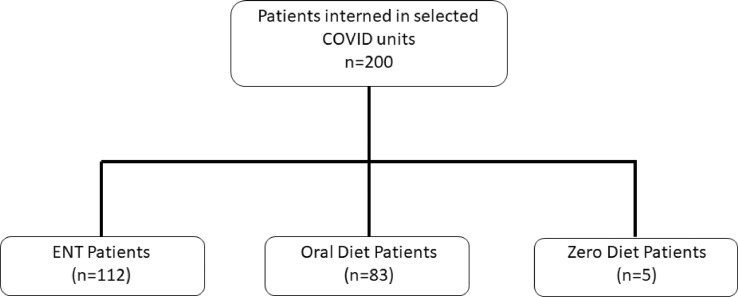
Considering the number of hospital beds, a 5% sampling error, and a 95% confidence interval (CI), the minimum sample size for this study was 100 patients. For this study, 112 patients on ENT were analyzed, and patients who had suspended diets (0 and/or suspended diet: n = 5) were excluded even before starting the first meal due to hemodynamic instability (subsequently evolving to death by the 5th d of hospitalization). Patients receiving an exclusively oral route diet were excluded as well (n = 83; Fig. 1 ).
Fig. 1.
Patient selection flow chart
Information collection technique
The instrument used to collect data from the analyzed electronic medical records addressed sociodemographic, clinical, and nutritional aspects. Information on sex and age were collected as sociodemographic aspects. Clinically related variables were outlined as past pathologic history comorbidities (i.e., systemic arterial hypertension, diabetes mellitus, obesity, chronic obstructive pulmonary disease, heart disease, cancer, chronic kidney disease), previous treatment medications (i.e., angiotensin conversion enzyme inhibitors, angiotensin II receptor antagonists, nonsteroidal anti-inflammatory drugs and corticosteroids), respiratory support (i.e., mechanical ventilation, supplementary oxygen supply), and smoking status (i.e., smoker, former smoker, nonsmoker).
The medications used (i.e., antibiotics, ivermectin, oseltamivir, azithromycin, hydroxychloroquine, corticosteroids), use or not of the prone position, complications presented during hospitalization (i.e., cardiorespiratory arrest, sepsis, hyposaturation, pneumonia, shock, acute renal failure, hemodialysis, acute coronary syndrome), prophylactic drug use (i.e., anticoagulants), number of days hospitalized (i.e., wards and ICUs), and clinical outcomes (i.e., hospital discharge or death) were evaluated. The nutritional variables analyzed (i.e., period of hospitalization at ICUs) were access to nutritional therapy, days in therapy, energy and protein supply relationships to ideal body weight, total caloric value, distribution of macronutrients, and adjustments according to BRASPEN [15].
The cutoff point for choosing energy goals was the 4th d of ENT use. Due to restrictions on professional nutritionists in the COVID-19 treatment units, indications for ENT suggested in the literature could not be adapted [15,16]. The use of a calorimeter to measure and calculate patient energy needs was unavailable due to an absence of equipment and the risk of contamination. Fast predictive formulas were used to calculate the caloric goal according to ENT use days and in accordance with the national recommendations of BRASPEN 15 (15–20 kcal/kg/d until 4th d of ENT use, progressing to 25–30 kcal/kg/d from 4th d onward).
In the absence of current weight or anthropometric measures to calculate estimated body weight, the ideal body weight was derived from the following equation: Height² × average body mass index by age and sex [17]. The nutrition service of the hospital under study is in the process of implementing quality indicators. One of the indicators in critical patients is the offering of at least 80% of the daily energy goal as a goal. The percentage of diet adequacy was calculated by the relationship between total caloric value and total energy expenditure.
Data analysis
The statistical analysis was performed using the Statistical Package for the Social Sciences, version 20.0, and P < 0.05 was considered significant. For the analysis, the maximum values obtained from offered energy and protein, as well as their relationships with body weight, total caloric value, distribution of macronutrients, and various adaptations during the hospitalization period, were used. Demographic variables, smoking habits, diseases, use of previous medications, drug therapy, breathing, complications during hospitalization, and outcomes were presented in absolute and relative values. Continuous variables were presented as means and standard deviation (SD), or median and 25th and 75th percentiles. Adherence to the normal curve was evaluated using the Kolmogorov–Smirnov test.
Beta estimates and 95% CI associations between outcomes and prone positioning with nutritional variables were analyzed using a linear regression adjusted for sex and age. Odds ratio (OR) and 95% CI estimates for a death outcome were analyzed by logistic regression adjusted for sex, age, systemic arterial hypertension, cardiorespiratory arrest, shock, acute renal failure or hemodialysis, use of corticosteroids during hospitalization, use of the prone position, and mechanical ventilation. For the association between use of the prone position or death and characteristics of nutritional therapy, a multiple linear regression model with forward strategy was used. The selection of covariates was based on the literature or when P < 0.20 in the univariate analysis.
Results
Of the 112 patients evaluated, the majority were male (n = 61; 54.5%), elderly (n = 88; 78.6%), and currently not smoking (n = 81; 72.3%). Of the preexisting diseases and risk factors, the most common were hypertensive (n = 72; 64.3%), diabetic (n = 44; 39.3%), and heart disease (n = 38; 33.9%). The most common continuous-use drugs were angiotensin conversion enzyme inhibitors (n = 39; 34.8%) and angiotensin II receptor antagonists (n = 44; 39.2%). Most patients died during hospitalization (n = 98; 87.5%). The median hospital stay was 14 d, with most in the ICU (median: 9 d; Table 1 ).
Table 1.
Description of sample
| Demographic variables | n | % |
|---|---|---|
| Sex | ||
| Female | 51 | 45.5 |
| Male | 61 | 54.5 |
| Age, y | ||
| <60 | 24 | 21.4 |
| ≥60 | 88 | 78.6 |
| Smoking | ||
| No | 81 | 72.3 |
| Smoke or former smoker | 31 | 27.7 |
| Diseases and preexisting risk factors | ||
| Systemic arterial hypertension | 72 | 64.3 |
| Diabetes mellitus | 44 | 39.3 |
| Dyslipidemia | 9 | 8.0 |
| Obesity | 15 | 13.4 |
| Cancer | 8 | 7.1 |
| Chronic kidney disease | 7 | 6.3 |
| Chronic obstructive pulmonary disease | 14 | 12.5 |
| Heart disease | 38 | 33.9 |
| Use of preexisting medications | ||
| Angiotensin conversion enzyme inhibitors | 39 | 34.8 |
| Angiotensin II receptor antagonists | 44 | 39.2 |
| Nonsteroidal antiinflammatory drugs | 4 | 3.6 |
| Corticosteroids | 18 | 16.1 |
| Clinical outcomes | ||
| Hospital discharge | 14 | 12.5 |
| Death | 98 | 87.5 |
| Days of hospitalization | Median | p25–p75 |
| Hospital days | 14 | 9–25 |
| Days in intensive care unit | 9 | 7–19 |
| Days in ward | 2 | 0–8 |
| Nutritional support data | Mean | Standard deviation |
| kcal/kg | 19.3 | 7.4 |
| protein/kg | 1.2 | 0.5 |
| Percentage of diet adequacy | 74.2 | 26.8 |
| Total caloric value, kcal | 1194.2 | 465.7 |
| Carbohydrate, % | 43.1 | 2.3 |
| Protein, % | 24.1 | 3.9 |
| Lipids, % | 32.9 | 1.8 |
Information on enteral nutritional support is presented in Table 1. The mean and SD for kcal/kg, protein/kg, and percentage of diet adequacy were 19.3 (SD: 7.4) kcal/kg, 1.2 (SD: 0.5) g/kg, and 74.2% (SD: 26.8%), respectively. According to the national guidelines for critically ill patients [16], the enteral route is preferable whenever the digestive tract is functioning, and supplemental parenteral nutrition is indicated for patients who cannot reach at least 65% of the caloric goal between the 5th and 7th day of enteral feeding. Our research showed that 15% of patients (n = 31) who received nutrition enterally received <65% of their daily energy needs and of these patients, 48% (n = 15) used the enteral route for <1 wk.
A full 29% of patients (n = 9) died before 1 wk of ENT, and 12.9% (n = 4) suffered breaks in enteral feeding due to complications or hospital procedures. A mixed diet (oral + enteral) was received by 9.6% of patients (n = 3). No patient used supplemental parenteral nutrition. In accordance with the national recommendations, all patients started ENT early, between 24–48 h after achieving hemodynamic stability [16]. Polymeric formulas were used for 99.1% of patients (n = 111), all of which had hypercaloric and hyperprotein characteristics.
The main complications during hospitalization were arterial oxyhemoglobin hyposaturation (n = 92; 82.1%), cardiorespiratory arrest (n = 54; 48.2%), shock (n = 51; 45.5%), and renal failure during hemodialysis (n = 45; 40.2%). The main drugs used during hospitalization were antibiotics (n = 111; 99.1%), anticoagulants (n = 107; 95.5%), and corticosteroids (n = 83; 74.1%; Table 2 ). Mechanical ventilation (89.3%) and supplemental oxygen (67.9%) were also recorded.
Table 2.
Drug therapy, respiratory therapy, and complications during hospitalization
| n | % | |
|---|---|---|
| Complications during hospitalization | ||
| Cardiorespiratory arrest | 54 | 48.2 |
| Sepsis | 26 | 23.2 |
| Hyposaturation | 92 | 82.1 |
| Pneumonia | 25 | 22.3 |
| Shock | 51 | 45.5 |
| Acute coronary syndrome | 20 | 17.9 |
| Acute renal failure or hemodialysis | 45 | 40.2 |
| Drug therapy during hospitalization | ||
| Use of anticoagulant | 107 | 95.5 |
| Antibiotic | 111 | 99.1 |
| Oseltamivir | 27 | 24.1 |
| Corticosteroids | 83 | 74.1 |
| Azithromycin | 83 | 74.1 |
| Ivermectin | 21 | 18.8 |
| Hydroxychloroquine | 35 | 31.3 |
| Respiratory support | ||
| Mechanical ventilation | 100 | 89.3 |
| Supplementary oxygen supply | 76 | 67.9 |
The use of the prone position impacted nutritional therapy. In general, patients in the prone position presented lowered amounts of kcal/kg of body weight, protein/kg of body weight, percentage of diet adequacy, and total caloric value (Table 3 ). The mean differences adjusted for sex and age between the groups were for kcal/kg (beta: –8.0; 95% CI, –10.3 to –5.7), protein/kg (beta: –0.5; 95% CI, –0.6 to –0.3), dietary adequacy percentage (beta: –27.5; 95% CI, –36.1 to –19.0), and total caloric value (beta: –458.1; 95% CI. –614.0 to –302.3).
Table 3.
Differences in nutritional therapy according to prone position performance
| Characteristics of nutritional therapy | Use of prone position |
Beta* | 95% confidence interval | |
|---|---|---|---|---|
| No (n = 58) | Yes (n = 54) | |||
| kcal/kg of weight | 23.4 (5.7) | 14.9 (6.4) | –8.0 | –10.3 to –5.7 |
| Protein/kg of weight | 1.4 (0.4) | 0.9 (0.4) | –0.5 | –0.6 to –0.3 |
| Percentage of diet adequacy | 88.3 (20.7) | 59.1 (24.4) | –27.5 | –36.1 to –19.0 |
| Total caloric value, kcal | 1416.1 (395.9) | 955.8 (417.0) | –458.1 | –614.0 to –302.3 |
| Carbohydrate, % | 43.2 (1.9) | 43.0 (2.7) | –0.2 | –1.1 to 0.7 |
| Protein, % | 24.1 (3.1) | 24.2 (4.7) | 0.1 | –1.4 to 1.7 |
| Lipids, % | 32.8 (1.2) | 32.9 (2.3) | 0.1 | –0.6 to 0.8 |
Linear regression adjusted for sex and age. Bold values are statistically significant.
Patients who died presented lower average maximum kcal/kg of body weight, protein/kg of body weight, percentage of diet adequacy, and total dietary caloric values than patients who were discharged from the hospital (Table 4 ). Average differences adjusted for sex and age between the groups were kcal/kg (beta: –5.5; 95% CI, –9.5 to –1.5), protein/kg (beta: –0.3; 95% CI, –0.6 to –0.02), dietary adequacy percentage (beta: –14.9; 95% CI, –29.6 to –0.15), and total caloric value (beta: –420.7; 95% CI, –675.0 to –166.4).
Table 4.
Differences in nutritional therapy according to outcome
| Characteristics of nutritional therapy | Outcome |
Beta* | 95% confidence interval | |
|---|---|---|---|---|
| Hospital discharge (n = 14) | Death (n = 98) | |||
| kcal/kg of weight | 23.8 (5.1) | 18.7 (7.5) | –5.5 | –9.5 to –1.5 |
| Protein/kg of weight | 1.4 (0.3) | 1.2 (0.5) | –0.3 | –0.6 to –0.02 |
| Percentage of diet adequacy | 86.6 (15.7) | 72.5 (27.7) | –14.9 | –29.6 to –0.15 |
| Total caloric value, kcal | 1562.1 (341.9) | 1141.6 (458.4) | –420.7 | –675.0 to –166.4 |
| Carbohydrate, % | 43.2 (1.7) | 43.1 (2.4) | –0.2 | –1.5 to 1.2 |
| Protein, % | 24.0 (2.8) | 24.1 (4.1) | 0.2 | –2.1 to 2.4 |
| Lipids, % | 32.8 (1.0) | 32.9 (1.9) | 0.1 | –1.0 to 1.1 |
Linear regression adjusted for sex and age. Bold values are statistically significant.
The association between death and ENT data adjusted for confounding factors was evaluated (Table 5 ). Patients on an enteral diet of ≥25 kcal/kg of weight/d presented with a lower OR compared with patients who did not reach at least 25 kcal/kg of weight/d (OR: 0.14; 95% CI, 0.02–0.86). In addition, patients on an enteral diet of ≥1.2 protein/kg of weight presented with a lower OR compared with patients who did not reach at least 1.2 protein/kg of weight/d (OR: 0.10; 95% CI, 0.01–0.97). Percentage of diet adequacy was not associated with death.
Table 5.
Association between death and adequacy of nutritional therapy
| Adequacy of nutritional therapy | Outcome |
Odds ratio* | 95% confidence interval | |
|---|---|---|---|---|
| Hospital discharge | Death | |||
| kcal/kg of weight | ||||
| <25 kcal/kg of weight/d | 6 (42.9%) | 77 (78.6%) | Reference | |
| ≥25 kcal/kg of weight/d | 8 (57.1%) | 21 (21.4%) | 0.14 | 0.02–0.86 |
| Protein/kg of weight | ||||
| <1.2 protein/kg of weight/d | 3 (21.4%) | 46 (46.9%) | Reference | |
| ≥1.2 protein/kg of weight/d | 11 (78.6%) | 52 (53.1%) | 0.10 | 0.01–0.97 |
| Percentage of diet adequacy | ||||
| <80% of goal | 6 (42.9%) | 53 (54.1%) | Reference | |
| ≥80% of goal | 8 (57.1%) | 45 (45.9%) | 0.54 | 0.84–3.51 |
Logistic regression adjusted for sex, age, systemic arterial hypertension, cardiorespiratory arrest, shock, acute renal failure or hemodialysis, use of corticosteroids during hospitalization, use of prone position, and mechanical ventilation. Bold values are statistically significant.
Discussion
The results of this study show that the population affected by COVID-19 in this study were mostly elderly, male, and had systemic arterial hypertension. The findings corroborate research conducted in China, the United States, and Italy describing the profile of patients hospitalized for COVID-19 during the first wave of infections [18], [19], [20].
A systematic review composed of 45 studies evidenced a prolonged hospitalization period for this pathology during the year 2020, with a median ranging from 4 d to 53 d in China and 4 d to 21 d in countries other than China. Data from eight studies referring to the length of hospital stay in the ICU reveal a median of 6 d to 12 d within China and 4 d to 19 d outside of China [21]. The data characterizing samples and lengths of hospital stay are similar to those from other countries, strengthening the comparisons with our findings.
Our results regarding enteral nutritional support showed that most of the individuals studied received inadequate caloric input. Pironi et al. [22] showed that the more dependent patients are on invasive oxygen support, the worse the energy adequacy is, and both their and our studies revealed suboptimal intakes compared with the recommendations of European Society for Clinical Nutrition and Metabolism and BRASPEN [11,12]. The energy and protein offered in Italian research presents a prescription of 26.5 kcal/kg/d and 1.1 g/protein/kg/d [22]; thus, offering more energy and less protein than our findings.
Patients in the supine position received more calories and protein per kilogram of body weight, revealing the difficulties faced by the nutrition service in feeding patients in the prone position. A study in Spain evaluated adverse nutritional effects on patients in the prone position. Of the 33 patients analyzed, 29 received enteral nutrition without complications and only four patients presented with vomiting. However, the study did not report quantitative data on the prescribed diet [23]. La Fuente et al. [24] found no significant volume differences in enteral nutrition-infused patients in the prone position compared with patients in the supine position. These findings do not corroborate those from our study. Aguila et al. [25] emphasizes the use of routine supplemental parenteral nutrition for patients in the prone position who are unable to reach the planned energy goal. The authors also highlight the importance of using parenteral nutrition to minimize the risk of probe displacement during pronation maneuvers.
Our sample followed the protocol guidelines for pronation, keeping the patient in reverse Trendelenburg at 20°, and following the enteral diet breaks recommended before and after performing the prone maneuver, in addition to maintaining a trophic diet throughout the pronation period [12,26]. The prone position generates a change in respiratory mechanics and benefits patients with SARS through improved oxygenation [26] despite studies revealing the challenge of properly and safely feeding these individuals. A recent publication by the American Society for Parenteral and Enteral Nutrition [27] compared different strategies for patients in the prone position: Routine gastric waste, tube positioning, and use of prokinetics. The authors suggested that the gastric residual volume should not be measured routinely in critically ill patients [28], and emphasized that the tube may be located in the stomach or small intestine because for patients in rotational therapy, its location does not alter the risk of microaspiration [29].
The use of prokinetic agents should be considered to accelerate gastric emptying and prevent vomiting [30], and hospital services routinely use prokinetics with all pronated patients. Reignier et al. [31] concluded that enteral nutrition is poorly tolerated in critically ill patients receiving invasive mechanical ventilation in the prone position, suggesting a reduction in infused volume while the patient is pronated. Data from the beginning of the outbreak in China revealed a high mortality rate among individuals who were severely affected by the disease. Hospital mortality was 97% in patients who progressed to mechanical ventilation, which may reflect the greater proportion of patients admitted in the early stages of the outbreak who were already severely affected [32].
In another report from Wuhan, mortality was 62% for critically ill patients infected with COVID-19 and 81% among patients requiring invasive ventilator support [33]. These data are similar to our findings on lethality. Our study found an association between protein and energy supply inadequacies and mortality risk. A study carried out with patients on enteral nutrition compared achievement of energy and protein goals and mortality, and showed that patients on enteral nutrition for >7 d and receiving higher daily amounts of proteins and calories per kilogram also presented with a lower mortality rate compared with patients with a less than adequate protein supply [34]. These data corroborate our findings, emphasizing the importance of optimizing the protein and energetic supply during enteral nutrition.
In a recent publication, de Watteville et al. [35] bring forward the association between nutritional parameters and the prognosis and outcome of patients hospitalized with COVID-19. A reduction in food intake before hospitalization as predicted by physicians during hospitalization is directly associated with worsening clinical outcomes. Thus, our findings note a high percentage of mortality, demonstrating the level of morbidity and mortality of the patients who sought medical help. This severity is directly related to hemodynamic instability, which is one of the main reasons for suspending food or feeding a trophic diet. Therefore, the malnutrition noted in our findings, in addition to being associated with the prone position, is also related to the severity of the disease.
This research brings contributions to the scientific community regarding the importance of nutritional assistance for patients infected with COVID-19 and the importance of monitoring enteral nutrition with the goal of achieving protein and energy targets that can minimize COVID-19’s intensified catabolism while avoiding a worsening prognosis.
Due to the absence of a nutritionist on the frontline at the study site during the peak of the pandemic in 2020, the anthropometric data used in the present study were based on electronic medical records, which is the study's principal limitation. Further studies are needed to optimize strategies and validate protocols involving nutritional assistance when combating COVID-19.
Conclusions
Our research confirms the importance of individualized therapeutic monitoring of enteral nutrition in individuals infected with COVID-19. A prolonged hospital stay and the pathophysiology of the disease lead to a state of nutritional depletion that compromises the patient's prognosis. The use of the prone position affected nutritional therapy in general and by body weight, patients in the prone position presented with lower maximum kcal/kg, protein/kg, percentage of diet adequacy, and total caloric values. Patients who died had lower average maximum kcal/kg, protein/kg, percentage of diet adequacy, and total caloric values compared with patients who survived and were discharged. There was an association between inadequacies in protein and energy supply with the risk mortality.
ALENCAR, ES; MUNIZ, LSS; HOLANDA, JLG; OLIVEIRA, BDD; CARVALHO MCF; LEITÃO, AMM; CARVALHO, MCF; CAVALCANTE, MIA; OLIVEIRA, RCP: was responsible for data collection, data interpretation, and article writing. SILVA, CAB: Intellectual contribution to the critical review of the article. CARIOCA, AAF: the design of the present study, analysis and interpretation of data, and main responsible for the review and approval of the final content. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
National Council for Scientific and Technological Development (Processes 422721/2018-2). No potential conflicts of interest were reported by the authors.
References
- 1.Laviano A, Koverech A, Manetti M. Nutrition support in the time of SARS-CoV-2 (COVID-19) Nutrition. 2020;74 doi: 10.1016/j.nut.2020.110834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang L, Liu Y. Potential interventions for novel coronavirus in China: A systematic review. J Med Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Souza de Cunha S, Araujo Santiago SA, de Carvalho Guedine CR, Sena Padua C, Rezende Meadow P. Nutrition therapy in adult patients with COVID-19: Scope review. BRASPEN J. 2021;1:93–100. [Google Scholar]
- 4.Artal FJC. Complicaciones neurológicas por coronavirus y COVID-19. Rev Neurol. 2020;70:311. doi: 10.33588/rn.7009.2020179. [DOI] [PubMed] [Google Scholar]
- 5.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J Pediatr. 2020;87:281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Coronavirus disease (COVID19) pandemic. Available at: https://www.who.int/health-topics/coronavirus. Accessed May 19, 2020.
- 7.Brasilia Ministério da Saúde, Secretaria de Vigilância em Saúde, Centro de Operações de Emergência em Saúde Boletim epidemiológico. Available at: https://www.saude.gov.br/boletins-epidemiologicos. Accessed May 19, 2020.
- 8.Metha S. Nutritional status and COVID-19: An opportunity for lasting change? Clin Med. 2020;20:270–273. doi: 10.7861/clinmed.2020-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Severin R, Arena R, Lavie CJ, Bond S, Phillips SA. Respiratory muscle performance screening for infectious disease management following COVID-19: A highly pressurized situation. Am J Med. 2020:347–348. doi: 10.1016/j.amjmed.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cena H, Maffoni S, Braschi V, Brazzo S, Pallavicini C, Vietti I, et al. Position paper of the Italian association of medical specialists in dietetics and clinical nutrition (ANSISA) on nutritional management of patients with COVID-19 disease. Med J Nutr Metab. 2020;13:1–5. [Google Scholar]
- 11.Barazzoni R, Bischoff SC, Breda J, Wickramasinghe K, Krznaric Z, Nitzan D, et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39:1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campos LF, Barreto PA, Ceniccola GD, Gonçalves RC, Matos LBN, Zambelli CMSF, et al. Revisão do parecer BRASPEN de terapia nutricional em pacientes hospitalizados com COVID-19. BRASPEN J. 2021;36:122–126. [Google Scholar]
- 13.Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7:4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castro MC, Kim S, Barberia L, Freitas Ribeiro A, Gurzenda S, Braga Ribeiro K, et al. Spatiotemporal pattern of COVID-19 spread in Brazil. Science. 2021;372:821–826. doi: 10.1126/science.abh1558. [DOI] [PubMed] [Google Scholar]
- 15.Castro MG, Ribeiro PC, Souza IAO, Cunha HFR, Silva MHN, Rocha EEM, et al. Brazilian guideline on nutritional therapy in critically patients. BRASPEN J. 2018;33:2–36. [Google Scholar]
- 16.Campos LF, Barreto PA, Ceniccola GD, Gonçalves RC, Matos LBN, Zambelli CMSF, et al. Parecer BRASPEN/AMIB para o enfrentamento do COVID-19 em pacientes hospitalizados. Apoio institucional da Associação de Medicina Intensiva Brasieira (AMIB) BRASPEN J. 2020;35:3–5. [Google Scholar]
- 17.Burr ML, Phillips MK. Antropometric norms in the elderly. Br J Nutr. 1984;51:165–169. doi: 10.1079/bjn19840020. [DOI] [PubMed] [Google Scholar]
- 18.Grasselli G, Zandrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu A, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rees EM, Nightingale ES, Jafari Y, Waterlow NR, Clifford S, Pearson CAB, et al. COVID-19 length of hospital stay: A systematic review and data synthesis. BMC Med. 2020;18:2–22. doi: 10.1186/s12916-020-01726-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pironi L, Sasdelli AS, Ravaioli F, Baracco B, Battaiola C, Bocedi G, et al. Malnutrition and nutritional therapy in patients with SARS-CoV-2 disease. Clin Nutr. 2021;40:1330–1337. doi: 10.1016/j.clnu.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodríguez-Huerta MD, Díez-Fernández A, Rodríguez-Alonso MJ, Robles-González M, Martín-Rodríguez M, González-García A. Nursing care and prevalence of adverse events in prone position: Characteristics of mechanically ventilated patients with severe SARS-CoV-2 pulmonary infection. Nurse Crit Care. 2021:1–8. doi: 10.1111/nicc.12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saez de la Fuente I, Saez de la Fuente J, Quintana Estelles MD, Garcia Gigorro R, Terceros Almanza LJ, Sanchez Izquierdo JA, et al. Enteral nutrition in patients receiving mechanical ventilation in a prone position. JPEN J Parenter Enteral Nutr. 2016;40:250–255. doi: 10.1177/0148607114553232. [DOI] [PubMed] [Google Scholar]
- 25.Aguila EJT, Cua IAY, Fontanilla JAC, Yabut VLM, Causing MFP. Gastrointestinal manifestations of COVID-19: Impact on nutrition practices. Nutr Clin Pract. 2020;35:800–805. doi: 10.1002/ncp.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliveira VM, Piekala DM, Deponti GN, Batista DCR, Minossi SD, Chisté M, et al. Checklist da prona segura: construção e implementação de uma ferramenta para realização da manobra de prona. Rev Bras Ter Intensiva. 2017;29:131–141. doi: 10.5935/0103-507X.20170023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behrens S, Kozeniecki M, Knapp N, Martindale RG. Nutrition support during prone positioning: An old technique reawakened by COVID-19. Nutr Clin Pract. 2021;1:105–109. doi: 10.1002/ncp.10592. [DOI] [PubMed] [Google Scholar]
- 28.Van der Voort PHJ, Zandstra DF. Enteral Feeding in the Critically Ill: Comparison Between the Supine and Prone Positions. A Prospective Crossover Study in Mechanically Ventilated Patients. Crit Care. 2001;5(4):216–220. doi: 10.1177/0115426502017005323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sams VG, Lawson CM, Humphrey CL, Brantley SL, Schumacher LM, Karlstad MD, et al. Effect of rotational therapy on aspiration risk of enteral feeds. Nutr Clin Pract. 2012;27:808–811. doi: 10.1177/0884533612462897. [DOI] [PubMed] [Google Scholar]
- 30.Reignier J, Dimet J, Martin-Lefevre L, Bontemps F, Fiancette M, Clementi E, et al. Before-after study of a standardized ICU protocol for early enteral feeding in patients turned in the prone position. Clin Nutr. 2010;29:210–216. doi: 10.1016/j.clnu.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Reignier J, Mercier E, Le Gouge A, Boulain T, Desachy A, Bellec F, et al. Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: A randomized controlled trial. JAMA. 2013;309(3):249–256. doi: 10.1001/jama.2012.196377. [DOI] [PubMed] [Google Scholar]
- 32.Weiss P, Murdoch DR. Clinical course and mortality risk of severe COVID-19. Lancet. 2020;395:1014–1015. doi: 10.1016/S0140-6736(20)30633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lew CCH, Wong GJY, Cheung KP, Fraser RJL, Chua AP, et al. The association between nutritional adequacy and 28-day mortality in the critically ill is not modified by their baseline nutritional status and disease severity. Crit Care. 2019;23:222. doi: 10.1186/s13054-019-2500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Watteville A, Montalbano F, Wozniak H, Collet TH, Jaksic C, Le Terrier C, et al. Impact of nutritional therapy during the first wave of the COVID-19 pandemic in intensive care patients: A retrospective observational study. Clin Nutr. 2021;S0261-5614(21) doi: 10.1016/j.clnu.2021.05.024. 00274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]