Abstract
Background
Coronavirus disease 2019 (COVID-19) had become a worldwide health threat. Early prediction of the severity of COVID-19 patients was important for reducing death rate and controlling this disease.
Methods and materials
A total of 301 patients confirmed with COVID-19 in Wuhan from 8 February to 10 April 2020 were included. Clinical data were collected and analyzed. Diagnostic and prognostic utility of blood cell counts and lymphocyte subsets in COVID-19 patients were investigated. The receiver operator characteristic curve (ROC) was used in discriminating the mild and severe/critical cases.
Results
There were difference in blood cell counts and lymphocyte subsets among mild, severe and critical patients, which were also influenced by comorbidities and duration of disease. The area under the ROC of lymphocyte, CD3+ T cells, CD4+ T cells, and CD8+ T cells were 0.718, 0.721, 0.718, and 0.670, which were higher than that of other hematological parameters. The optimal threshold was 1205, 691, 402, and 177 per μl, respectively. Patients with higher counts of lymphocyte, CD3+ T cells, CD4+ T cells, or CD8+ T cells were correlated with shorter length of stay in hospital (p < 0.05). Multivariable Cox regression analysis showed disease severity, CD3+ T cells counts and time when the nucleic acid turned negative were independent risk factors for in-hospital death of COVID-19 patients (p < 0.05).
Conclusion
Blood cell counts and lymphocyte subsets correlated with severity of COVID-19.
Keywords: COVID-19, lymphocyte subsets, severity, prognosis, risk factors
Introduction
COVID-19, which was caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), became pandemic throughout the world since March 2020. 1 Primarily manifested as a respiratory tract infection, most COVID-19 patients exhibit mild to moderate common symptoms including fever, cough, myalgia, or fatigue.2,3 Some patients are also accompanied by minor symptoms such as skin eruption, dysgeusia, headache, and gastrointestinal symptoms.4,5 Moreover, abnormalities could frequently be observed in blood and biochemistry tests, which indicate SARS-CoV-2 infection has a broad influence on multiple systems including cardiovascular, gastrointestinal, hematopoietic, and immune system.6,7 It was estimated about 15% patients may develop to severe pneumonia and approximately 5% eventually progress to acute respiratory distress syndrome, septic shock, and/or multiple organ failure.8,9
Accumulating data show lymphocytopenia and leukopenia are common in COVID-19 patients, the elevated neutrophil-to-lymphocyte ratio (NLR) might be correlated with severe disease10,11 and a low lymphocyte count might be associated with an increased risk of death. 12 It was observed CD4+ T and CD8+ T cells were reduced after infection, which had greater reductions in the severe patients than those in the mild patients. 13 Moreover, decreased lymphocyte subsets improved before patients were discharged from hospital. 14 The immune response activated by SARS-CoV-2 infection was crucial for the clearance of invading pathogens. Uncontrolled inflammatory innate immune responses and impaired adaptive immune responses could cause harmful tissue damage. 15 It was of utmost importance to delineate changes of immune function in COVID-19 patients and look for useful treatment to rebuild immune balance.
Previous studies indicated the changes of peripheral lymphocytes in COVID-19 patients; however, the alternation in lymphocyte subsets and its prognostic value remain unclear. In the present study, we aimed to describe the characteristic of blood cell counts and lymphocyte subsets in patients with COVID-19. The diagnostic and prognostic utility of these parameters were also evaluated, which might help to elucidate the pathogenesis of COVID-19 and provide clues for developing novel biomarkers and therapeutic strategies.
Materials and methods
Patients and data collection
This was a retrospective study among patients recruited from Huoshenshan Hospital, which was a hospital specialized for the treatment of COVID-19 patients in Wuhan, China. The research was approved by Ethnic Committee of Huoshenshan Hospital and written informed consent was waived by the Ethnic Committee. Patients were included in the study when the following criteria were met: (1) over 18 years old; (2) diagnosed with COVID-19 pneumonia according to the criterion of Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (Seventh Edition, released by the National Health Commission of the China); (3) with adequate clinical information. Patients died within 3 days after hospitalization or lack essential information for analysis were excluded from the study. From February 8 to 10 April 2020, 301 consecutive patients with COVID-19 admitted in Huoshenshan Hospital were enrolled in this study. Patient information including epidemiological, clinical, laboratory and radiological characteristics, treatment and outcomes were manually collected from electronic records. Data reliability was checked by two researchers independently. Sample size was calculated and justified as preciously described. 16
Confirmation of SARS-CoV-2 infection
All patients were confirmed with SARS-CoV-2 infection with a positive result on real-time reversetranscriptase-polymerase-chain-reaction (RT-PCR) assay of nasal and pharyngeal swab specimens, according to the criterion of Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (Seventh Edition).
Severity of COVID-19 patients
The degree of severity (mild, severe, and critical cases) was classified by using the mentioned guidance, based on results from chest radiography, clinical examination, and symptoms. Patients with mild symptoms (fever, cough, expectoration, and other upper respiratory tract symptoms), and without abnormalities, or with mild changes on chest radiography, were classified as mild cases. Severe cases were defined as meeting one of these conditions: (1) respiratory rate ≥30 breaths/min; (2) arterial oxygen tension (PaO2) over inspiratory oxygen fraction (FIO2) of less than 300 mmHg; (3) Percutaneous oxygen saturation (SpO2) ≤93% on room air at rest. Critical cases were defined as on the following conditions: (1) occurrence of severe respiratory distress; (2) respiratory failure requiring mechanical ventilation; (3) shock and organ failure even needs of ICU care.
Lymphocyte subsets detection
Lymphocyte subsets were detected by using flow cytometry. Peripheral blood samples were collected from COVID-19 patients and analyzed within 6 h after being obtained. A 100 μL of peripheral whole blood sample was labeled with antibodies including anti-CD3-fluorescein isothiocyanate (FITC), anti-CD4-phycoerythrin (PE), anti-CD45-peridin chlorophyll alpha protein (PerCP), and anti-CD8-allophycocyanin (APC). Another 100 μL of peripheral whole blood sample was labeled with antibodies including anti-CD3-FITC, anti-CD16 +56-PE, anti-CD45-PerCP, and anti-CD19-APC (BD Multitest). The samples were vortexed and kept at room temperature for 15 min in darkness. Then, each sample was analyzed using multiple-color flow cytometry (BD FACSCanto) according to the manufacturer’s instructions.
Statistical analysis
Categorical variables were described as frequency and percentages, and continuous variables as median and interquartile range (IQR). Differences in the levels of these parameters between the mild, severe, and critical patients of the disease were determined with the Mann–Whitney test. The area under the curve (AUC) and the 95% confidence interval (CI) of the ROC curve was computed using the predicted probability of severe COVID-19 pneumonia. The optimal cut‐off points to predict the severity of COVID‐19 were determined by Youden’s index. Kaplan–Meier analysis was used to investigate relationship between lymphocyte subsets and the length of stay. Multivariable Cox regression was used to explore the association between clinical features and the severity of disease. A two‐sided p value less than 0.05 was considered significant. The results of the analysis were obtained using SPSS 18.0 software.
Results
Demographics and baseline clinical characteristics
A total of 301 patients were included (median age, 60 years; range, 19–100 years; 47.2% female) (Table 1). At triage, 148 (49.1%) patients had pre-existing comorbidities, such as chronic obstructive pulmonary disease, cardiovascular disease, chronic liver disease or chronic kidney disease; 144 (47.8%) patients were admitted in hospital within 30 days after they had symptoms of infection. The mean length of stay was (15 ± 0.63) days. According to the categorical criteria, 186 (61.8%) were mild cases, 87 (28.9%) were severe ill, and 28 (9.3%) were critical ill. There were significant difference in age, sex, comorbidities, and length of stay among the mild, severe and critical ill group (p < 0.001). Compared to the mild cases, the severe or critical cases were older and lived longer in the hospital. (Table 1). All patients were treated according to national guidelines, 189 (62.8%) received antiviral treatment, 97 (32.2%) had been treated with glucocorticoids, and 83 (27.6%) had been treated with thymosin.
Table 1.
Clinical characteristics of patients with COVID-19.
| All patients n (%) | Disease severity | p value a | |||
|---|---|---|---|---|---|
| Mild n (%) | Severe n (%) | Critical n (%) | |||
| Age b | 60 (19–100) | 58 (19–90) | 65 (21–100) | 71 (49–89) | <0.001 |
| Sex | <0.001 | ||||
| Male | 159 (52.8) | 91 (48.9) | 50 (57.5) | 18 (64.2) | |
| Female | 142 (47.2) | 95 (51.1) | 37 (42.5) | 10 (35.7) | |
| Comorbidities | <0.001 | ||||
| Non | 153 (50.9) | 114 (61.3) | 36 (41.4) | 3 (10.7) | |
| Chronic obstructive pulmonary disease | 72 (23.9) | 39 (21.0) | 21 (24.1) | 12 (42.9) | |
| Cardiovascular disease | 31 (10.3) | 15 (8.1) | 13 (14.9) | 3 (10.7) | |
| Chronic liver disease | 27 (8.9) | 14 (7.5) | 9 (10.3) | 4 (14.3) | |
| Chronic kidney disease | 18 (6.0) | 4 (2.2) | 8 (9.2) | 6 (21.4) | |
| Legth of stay c | 15.13±0.63 | 12.59±0.54 | 16.93±1.39 | 26.46±3.02 | <0.001 |
| Antiviral treatment | <0.001 | ||||
| Yes | 189 (62.8) | 109 (58.6) | 60 (68.9) | 20 (71.4) | |
| No | 112 (37.2) | 77 (41.3) | 27 (31.0) | 8 (28.5) | |
| Use of glucocorticoid | <0.001 | ||||
| Yes | 97 (32.2) | 34 (18.3) | 40 (46.0) | 23 (82.1) | |
| No | 204 (67.7) | 152 (81.7) | 47 (54.0) | 5 (17.9) | |
| Use of thymosin | <0.001 | ||||
| Yes | 83 (27.6) | 30 (16.2) | 35 (40.2) | 18 (64.2) | |
| No | 218 (72.3) | 156 (83.8) | 52 (59.7) | 10 (35.7) | |
aComparison among mild, severe, and critical groups.
bData were presented as median (range), years.
cData were presented as mean±SD, days.
Full blood cell counts and lymphocyte subsets in COVID-19 patients
The median counts of white blood cells, neutrophils, lymphocytes and monocytes were 5480 (IQR, 4400–7490), 3550 (IQR, 27,060–5000), 1440 (IQR, 1020–1820), and 460 (IQR, 330–650) per μl (Table 2). Among the mild, severe, and critical groups, there was no significant difference in white blood cell counts (p = 0.065). However, there was significant difference in counts and percentages of neutrophils, lymphocytes, or monocytes (p = 0.001). There was a tendency that neutrophils increased and lymphocytes decreased with the aggravation of the disease. The median NLR in COVID-19 patients was 2.46 (IQR, 1.77–4.09). Neutrophil-to-lymphocyte ratio in the critical ill group increased to 8.78 (IQR, 4.63–17.61), which was significant higher than that in mild or severe group (p = 0.001) (Table 2).
Table 2.
Blood tests of patients with COVID-19.
| All patients (n = 301) | Disease severity | p value a | |||
|---|---|---|---|---|---|
| Mild (n = 186) | Severe (n = 87) | Critical (n = 28) | |||
| Leucocytes, per μl | 5480 (4400–7490) | 5300 (4450–7020) | 5620 (4280–8360) | 7280 (47,80–103,30) | 0.065 |
| Neutrophils, per μl | 3550 (2760–5000) | 3370 (2680–4450) | 3720 (2730–5330) | 6280 (4070–8120) | 0.001 |
| Neutrophil percentage, % | 64.1 (57.35–71.91) | 62.4 (57.25–67.50) | 63.6 (56.6–72.2) | 89.95 (83.85–94.15) | 0.001 |
| Lymphocytes, per μl | 1440 (1020–1820) | 1580 (1230–1890) | 1190 (890–1690) | 785 (530–900) | 0.001 |
| Lymphocyte percentage, % | 26.2 (19.35–33.30) | 27.8 (23.9–34.01) | 26.11 (16.45–33.82) | 4.65 (3.17–14.3) | 0.001 |
| Monocyte, per μl | 460 (330–650) | 470 (330–620) | 500 (390–780) | 240 (140–520) | 0.001 |
| Monocyte percentage, % | 7.9 (6.5–10.15) | 7.8 (6.5–10.0) | 9.1 (7.52–10.97) | 4.25 (2.02–5.51) | 0.001 |
| NLR | 2.46 (1.77–4.09) | 2.1 (1.66–3.04) | 2.95 (1.80–4.63) | 8.78 (4.63–17.61) | 0.001 |
Data were presented as median (IQR).
aComparison among mild, severe, and critical groups.
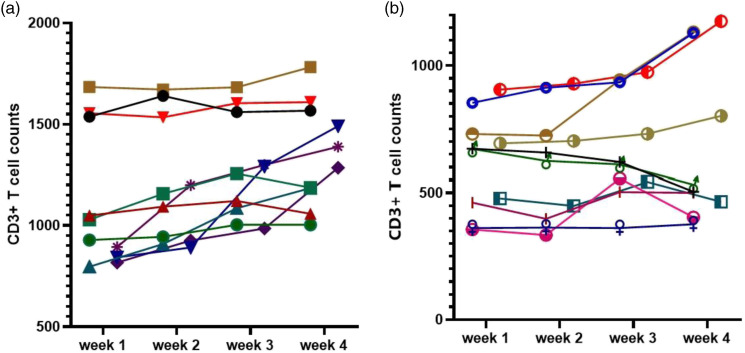
The median counts of CD3+ T cells, CD3+CD4+ T cells, CD3+CD8+ T cells, B cells (CD3−CD19+), and NK cells (CD3−CD16+CD56+) were 991.30 (IQR, 689.1–1234.2), 543.8 (IQR, 371.7–727.3), 323.2 (IQR, 205.3–449.9), 156.5 (IQR, 90.3–224.9), and 212.4 (IQR, 127.4–309.8) per μl, respectively. Moreover, statistical difference was found among groups of different disease severity. CD4+/CD8+ ratio was 1.7 (IQR, 1.3–2.5) in all COVID-19 patients. CD4+/CD8+ ratio increased to 2.7 (IQR, 2.2–3.1) in critical ill group, which was higher than that in mild or severe group (p = 0.001). Although the frequencies of B cells and NK cells showed no difference, the absolute counts of B cells and NK cells were statistically different among different groups of patients (p = 0.001) (Table 3). The lymphocyte subsets were analyzed every week in several patients during hospitalization, including 10 mild ill patients and 10 critical ill patients (Figure 1). For most of the patients, CD3+ T cells increased after treatment. However, CD3+ T cells did not change or even decreased in some critical cases.
Table 3.
Lymphocyte subsets of patients with COVID-19.
| All patients (n = 301) | Disease severity | p value a | |||
|---|---|---|---|---|---|
| Mild (n = 186) | Severe (n = 87) | Critical (n = 28) | |||
| CD3+ T cells, per μl | 991.3 (689.1–1234.2) | 1108.1 (813.2–1307.0) | 875.6 (630.0–1146) | 389.7 (280.1–610.0) | 0.001 |
| CD3+ T cells, % | 70.2 (62.65–75.80) | 70.7 (63.8–75.9) | 71.3 (63.5–76.7) | 61.2 (52.6–70.2) | 0.001 |
| CD4+ T cells, per μl | 543.8 (371.7–727.3) | 624.1 (453.1–802.0) | 449.0 (282.2–686.9) | 250 (206.0–379.0) | 0.001 |
| CD4+ T cells, % | 39.7 (33.1–46.65) | 40.6 (34.5–46.5) | 37.8 (29.7–46.4) | 41.1 (33.7–49.6) | 0.118 |
| CD8+ T cells, per μl | 323.2 (205.3–449.9) | 341.6 (251.5–462.1) | 313.0 (216.0–459.1) | 99.1 (66.1–167.1) | 0.001 |
| CD8+ T cells, % | 23.3 (18.15–29.1) | 22.8 (17.7–28.8) | 25.9 (21.6–31.3) | 17.2 (12.4–21.3) | 0.001 |
| CD4+/CD8+ | 1.7 (1.3–2.5) | 1.8 (1.3–2.5) | 1.6 (1.0–2.1) | 2.7 (2.2–3.1) | 0.001 |
| B cells, per μl | 156.5 (90.3–224.9) | 167.8 (113.9–233.1) | 132.2 (56.7–213.8) | 856.6 (53.2–190.8) | 0.001 |
| B cells, % | 10.7 (7.3–13.7) | 10.8 (7.6–13.5) | 10.3 (5.57–14.2) | 12.35 (7.3–26.5) | 0.185 |
| NK cells, per μl | 212.4 (127.4–309.8) | 237.0 (156.4–329.8) | 180.2 (109.1–290.6) | 655.1 (26.7–213.4) | 0.001 |
| NK cells, % | 16.0 (10.9–21.7) | 16.1 (11.45–21.6) | 16.3 (11.9–21.5) | 10.8 (5.4–24.3) | 0.279 |
Data were presented as median (IQR).
aComparison among mild, severe, and critical groups.
Figure 1.
Dynamic analysis of T cell counts in patients with COVID-19. (a) CD3+ T cell counts were monitored every week in 10 mild ill patients. (b) CD3+ T cell counts were monitored every week in 10 critical ill patients. Each line represents a patient.
Table 4 shows the influence of comorbidities on blood tests and lymphocyte subsets in COVID-19 patients. As shown in Table 4, lymphocyte counts were significant lower in patients with comorbidities. Neutrophil-to-lymphocyte ratio was similar among mild patients with or without comorbidities; however, NLR was significantly higher in severe/critical patients with comorbidities compared to patients without comorbidities (p = 0.001). CD3+ T cells, CD4+ T cells, CD8+ T cells, and CD4+/CD8+ ratio were higher in severe/critical patients without comorbidities compared to patients with comorbidities (p = 0.001). It was also found NK cell counts was increased in severe/critical patients with comorbidities (p = 0.001).
Table 4.
Blood tests and lymphocyte subsets of COVID-19 patients with or without comorbidities.
| Mild patients | Severe/Critical patients | |||||
|---|---|---|---|---|---|---|
| With comorbidities (n = 72) | Without comorbidities (n = 114) | p Value | With comorbidities (n = 76) | Without comorbidities (n = 93) | p Value | |
| Leucocytes, per μl | 5250 (4370–7040) | 5330 (4570–7010) | 0.648 | 6260 (4580–9510) | 5550 (4160–7410) | 0.121 |
| Neutrophils, per μl | 3370 (2750–4430) | 3310 (2610–4460) | 0.815 | 4520 (3200–7490) | 3580 (2770–4840) | 0.001 |
| Neutrophil percentage, % | 64.2 (5870–6970) | 61.4 (55.7–66.5) | 0.180 | 74.6 (60.3–85.1) | 63.3 (56.3–70.9) | 0.001 |
| Lymphocytes, per μl | 1440 (1120–1780) | 1640 (1260–1910) | 0.041 | 960 (650–1220) | 1420 (960–1800) | 0.001 |
| Lymphocyte percentage, % | 22.6 (22.4–33.2) | 29.3 (24.5–35.7) | 0.032 | 16.3 (7.95–29.0) | 26.6 (20.0–33.7) | 0.001 |
| Monocyte, per μl | 460 (310–600) | 470 (330–640) | 0.595 | 450 (310–760) | 460 (360–670) | 0.001 |
| Monocyte percentage, % | 7.90 (6.5–10.0) | 7.750 (6.5–9.9) | 0.733 | 7.9 (5.35–10.52) | 8.7 (7.4–10.9) | 0.001 |
| NLR | 2.28 (1.74–3.15) | 2.07 (1.57–3.03) | 0.210 | 4.45 (2–10.8) | 3.58 (2.77–4.84) | 0.001 |
| CD3+ T cells, per μl | 1000.4 (716.2–1228.7) | 1130.2 (922.4–1335) | 0.06 | 670.2 (360.2–921.9) | 983.6 (662.5–1215.9) | 0.001 |
| CD3+ T cells, % | 69.7 (59.7–75.0) | 71.4 (64.0–76.6) | 0.072 | 65.6 (58.4–75.9) | 70.7 (65.2–75.9) | 0.001 |
| CD4+ T cells, per μl | 563.2 (414.1–702.4) | 650.7 (486.0–844.3) | 0.09 | 326.8 (223.3–494.7) | 535.5 (387.5–771.6) | 0.001 |
| CD4+ T cells, % | 39.8 (32.2–44.2) | 41.3 (35.5–47.2) | 0.193 | 37.5 (29.0–44.7) | 40.8 (35.2–49.0) | 0.186 |
| CD8+ T cells, per μl | 317.6 (215.0–427.7) | 387.5 (277.1–508.9) | 0.07 | 213.5 (112.9–442.1) | 291.0 (218.4–442.4) | 0.001 |
| CD8+ T cells, % | 21.5 (16.9=28.7) | 23.3 (18.2–29.6) | 0.221 | 24.4 (17.3–30.6) | 24.3 (19.2–28.5) | 0.001 |
| CD4+/CD8+ | 1.9 (1.3–2.5) | 1.7 (1.3–2.5) | 0.699 | 1.6 (1.07–2.60) | 1.85 (1.37–2.7) | 0.001 |
| B cells, per μl | 146.6 (98.9–230.0) | 172.5 (122.1–234.4) | 0.063 | 93.2 (44.0–188.8) | 176.3 (94.7–244.2) | 0.448 |
| B cells, % | 9.4 (7.22–13.55) | 11.4 (8.0–13.5) | 0.285 | 9.75 (5.27–13.9) | 12.2 (9.0–15.6) | 0.072 |
| NK cells, per μl | 258.8 (165.8–356.7) | 231.1 (152.3–310.2) | 0.375 | 180.2 (77.7–285.6) | 140.3 (97.7–230.6) | 0.001 |
| NK cells, % | 16.95 (12.57–23.70) | 15.5 (11.0–20.1) | 0.053 | 17.9 (11.9–22.8) | 12.9 (8.0–16.7) | 0.195 |
Data were presented as median(IQR).
Table 5 shows the influence of disease duration on blood tests and lymphocyte subsets in COVID-19 patients. In mild patients, disease duration had little impact on blood tests. However, in severe/critical patients, neutrophils and NLR decreased, lymphocytes increased as the course of disease prolonged. Moreover, CD8+ T cell and NK cell counts were higher in patients with course of the disease longer than 30 days.
Table 5.
Lymphocyte subsets of COVID-19 patients with different course of the disease.
| Mild patients | Severe/Critical patients | |||||
|---|---|---|---|---|---|---|
| Course of the disease <30 days (n = 76) | Course of the disease ≥30 days (n = 110) | p Value | Course of the disease <30 days (n = 66) | Course of the disease ≥30 days (n = 49) | p Value | |
| Leucocytes, per μl | 5660 (4390–7550) | 5220 (4480–6890) | 0.163 | 6760 (4830–9740) | 5430 (4010–7480) | 0.029 |
| Neutrophils, per μl | 3550 (2750–4860) | 3230 (2610–4350) | 0.120 | 4750 (3300–7290) | 3430 (2380–5040) | 0.001 |
| Neutrophil percentage, % | 62.5 (57.3–69) | 62.4 (57–67.2) | 0.714 | 76.9 (62.6–87.5) | 60.8 (55.7–70.9) | 0.001 |
| Lymphocytes, per μl | 1450 (1090–1890) | 1640 (1350–1900) | 0.20 | 920 (580–1170) | 1420 (980–1810) | 0.001 |
| Lymphocyte percentage, % | 28.0 (22.4–33.3) | 27.5 (24.4–34.2) | 0.548 | 15.0 (4.7–26.5) | 27.8 (20.2–34.2) | 0.001 |
| Monocyte, per μl | 500 (340–680) | 430 (320–580) | 0.140 | 450 (300–750) | 450 (360–740) | 0.761 |
| Monocyte percentage, % | 8.4 (6.7–10) | 7.6 (6.475–10) | 0.470 | 7.6 (5.15–10.4) | 9.0 (7.3–10.9) | 0.025 |
| NLR | 2.45 (1.81–3.41) | 2.06 (1.51–2.86) | 0.08 | 4.88 (2.54–11.15) | 2.13 (1.63–4.09) | 0.001 |
| CD3+ T cells, per μl | 1011.3 (718–1273.5) | 1131 (860.1–1340.1) | 0.062 | 629.2 (355.4–815.2) | 957.6 (709.3–114.9) | 0.001 |
| CD3+ T cells percentage % | 71.4 (64.0–76.7) | 69.6 (63.7–75.6) | 0.399 | 67.8 (60.8–76.1) | 70.5 (62.6–75.8) | 0.684 |
| CD4+ T cells, per μl | 617.4 (443.4–795.8) | 637.5 (469.0–8176.2) | 0.419 | 332.1 (220.5–480.0) | 529.1 (291.1–688.2) | 0.005 |
| CD4+ T cells, % | 42.8 (37.9–48.5) | 39.15 (32.76–43.6) | 0.002 | 40.2 (33–48.9) | 37.1 (29–45.3) | 0.106 |
| CD8+ T cells, per μl | 294.1 (215.2–432.3) | 388.3 (283.5–5115.7) | 0.001 | 192.5 (109.4–364.6) | 335.8 (247.1–450) | 0.001 |
| CD8+ T cells, % | 21.5 (17–27.7) | 24.3 (18.9–30.8) | 0.029 | 22.3 (17.9–26.9) | 26.4 (19.4–31.5) | 0.076 |
| CD4+/CD8+ | 2.0 (1.4–2.8) | 1.6 (1.2–2.3) | 0.004 | 1.9 (1.35–2.7) | 1.6 (0.9–2.3) | 0.064 |
| B cells, per μl | 158.5 (91.8–234.4) | 171.9 (123.5–228.2) | 0.27 | 95.7 (43.5–190.8) | 153.5 (86.7–219.2) | 0.055 |
| B cells, % | 10.8 (8.1–13.5) | 10.7 (7.4–13.62) | 0.459 | 10.7 (5.65–14.0) | 11 (6.5–15.5) | 0.737 |
| NK cells, per μl | 194.1 (118.2–328.9) | 258.8 (182.0–339.8) | 0.007 | 134.6 (55.5–219.5) | 222 (119–327) | 0.002 |
| NK cells, % | 15.4 (10.5–21.8) | 10.7 (7.4–13.62) | 0.165 | 14.7 (6.2–22.3) | 16.6 (11.1–21.7) | 0.378 |
Data were presented as median (IQR).
Diagnostic and prognostic utility of lymphocyte subsets in COVID-19 patients
To investigate the values of lymphocyte subsets in the prediction of disease severity in COVID-19 patients, the ROC cure was used to analyze the early warning efficiency and the optimal prediction threshold. Mild cases were classified as non-severe pneumonia, severe or critical cases were classified as severe pneumonia. As shown in Table 6, the AUC of lymphocyte, CD3+ T cells, CD4+ T cells, and CD8+ T cells were 0.718, 0.721, 0.718, and 0.670, which were higher than AUC of other parameters and indicated they could better predict whether the COVID-19 patients were complicated with severe pneumonia. The optimal threshold was 1205, 691, 402, and 177 per μl, respectively (Table 6).
Table 6.
ROC curves to evaluate blood tests and lymphocytes subsets in the prediction of severity of patients with COVID-19.
| Variables | AUC | 95% CI | p Value | Cut-off (per μl) |
|---|---|---|---|---|
| Lymphocyte counts | 0.718 | 0.656–0.780 | <0.001 | 1205 |
| CD3+ T cell | 0.721 | 0.659–0.783 | <0.001 | 691 |
| CD4+ T cell | 0.718 | 0.655–0.780 | <0.001 | 402 |
| CD8+ T cell | 0.670 | 0.573–0.707 | <0.001 | 177 |
AUC: area under the curve; ROC: receiver operator characteristic.
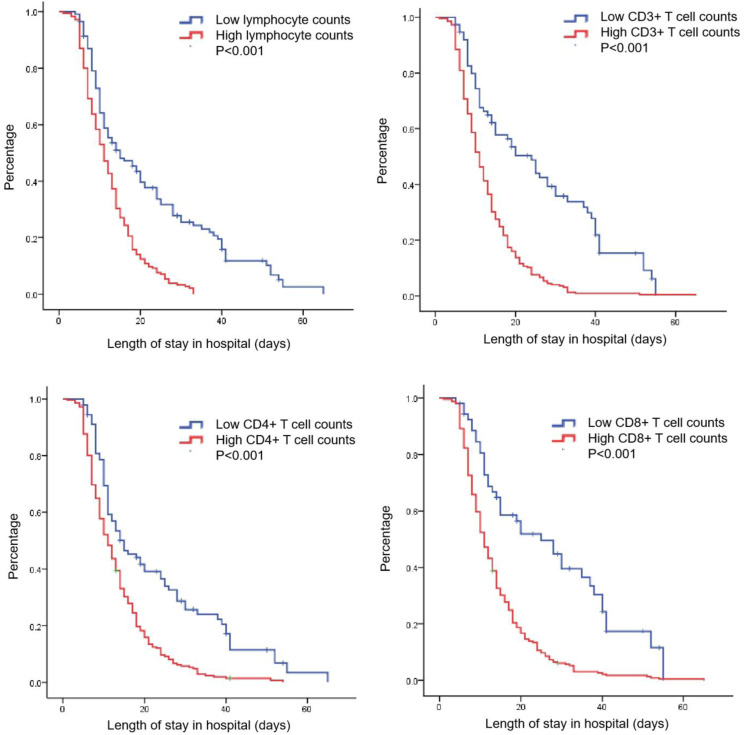
The relationship between lymphocyte subsets and the length of stay were also analyzed. Patients were divided into high and low groups based on optimal cut-off counts of lymphocyte, CD3+, CD4+, or CD8+ T cells mentioned above. It was found that patients with higher counts of lymphocyte, CD3+, CD4+, or CD8+ T cells were correlated with shorter length of stay in hospital (p < 0.001) (Figure 2).
Figure 2.
Lymphocyte subsets as a predictor of in hospital length of stay.
A Cox regression model was established to explore risk factors for in-hospital death of COVID-19 patients Table 7. It was found that disease severity, CD3+ T cells counts and time when the nucleic acid turned negative were independent risk factors that contributed to outcome of COVID-19 patients (p < 0.05). However, antiviral treatment and the use of glucocorticoids or thymosin had no significant impact on death of COVID-19 patients (p > 0.05).
Table 7.
Risk factors of in hospital death of patients with COVID-19.
| Variables | B | SE | Wald | Sig |
|---|---|---|---|---|
| Age | −0.010 | 0.005 | 3.631 | 0.057 |
| Sex | −0.012 | 0.126 | 0.009 | 0.924 |
| Severity | −0.413 | 0.154 | 7.202 | 0.007 |
| Comorbidities | 0.030 | 0.139 | 0.048 | 0.826 |
| Time when the nucleic acid turned negative | −0.530 | 0.147 | 12.997 | 0.000 |
| CD3+ T cell | −0.574 | 0.247 | 5.373 | 0.020 |
| CD4+ T cell | 0.194 | 0.206 | 0.893 | 0.345 |
| CD8+ T cell | −0.414 | 0.235 | 3.112 | 0.078 |
| Lymphocyte counts | −0.212 | 0.187 | 1.284 | 0.257 |
| Antiviral treatment | −0.147 | 0.282 | 0.271 | 0.602 |
| Glucocorticoid treatment | −0.221 | 0.284 | 0.608 | 0.436 |
| Thymosin treatment | −0.140 | 0.283 | 0.246 | 0.620 |
Discussion
The COVID-19 pandemic is worsening in severity globally. To further help our fight against COVID-19, it is of utmost importance to better clarify the clinical characteristics and identify prognostic biomarkers for patients at high risk of developing severe pneumonia.
SARS-CoV-2 infection could activate innate and adaptive immune responses. The immune response was essential to control and eliminate infections; however, maladjusted immune responses might result in immunopathology and impaired pulmonary gas exchange. 17 Lymphopenia was reported as a common feature in patients with COVID-19, indicating abnormal immune function during SARS-CoV-2 infection.18,19 Lymphocyte subsets played an important role in cellular immune regulation with each cell restricting and regulating each other. A number of studies had focused on characteristic of lymphocyte subsets in COVID-19 patients. Wan et al. 14 found there were significant differences in CD4+ T, CD8+ T, IL-6, IL-10, and PII between mild and severe COVID-19 patients. CD4+ T and CD8+ T in the severe group had greater reductions than those in the mild group. Xu et al. 20 also demonstrated increased inflammatory cytokines and suppressed T cell-mediated immunity in COVID-19. Moreover, they found lower counts of T lymphocyte subsets lymphocyte, CD3+ T cell, CD4+ T cell, CD8+ T cell, and B cell were associated with higher risks of in-hospital death of COVID-19.
In the present study, we described features of blood cell counts and lymphocyte subsets in COVID-19 patients. It was found that these blood parameters were different among mild, severe, and critical patients, which might also be influenced by comorbidities and duration of disease. To evaluate the diagnostic utility of lymphocyte subsets in predicting disease severity of COVID-19 patients, we used the ROC cure to analyze the early warning efficiency and the optimal prediction threshold. It was found that lymphocyte, CD3+, CD4+, and CD8+ T cells could better predict whether the COVID-19 patients were complicated with severe pneumonia. Based on optimal cut-off counts of lymphocyte subsets, we found that patients with higher counts of lymphocyte, CD3+, CD4+, or CD8+ T cells were correlated with shorter length of stay in hospital. Moreover, we demonstrated disease severity, CD3+ T cells counts, and time when the nucleic acid turned negative were independent risk factors that contributed to outcome of COVID-19 patients.
Since lymphocytopenia and T cell exhaustion was an important feature in COVID-19 patients, clinicians had tried many ways to reverse this imbalance. Thymosin Alpha 1 (Tα1) was a useful drug for the treatment of sepsis as an immune response modifier. 21 A recent study showed Tα1 treatment significantly reduces mortality of severe COVID-19 patients compared with untreated group (11.11% vs 30.00%, p = 0.044). 22 Tα1 could timely enhance blood T cell numbers in COVID-19 patients with severe lymphocytopenia. COVID-19 patients with lower counts of CD8+ T cells or CD4+ T cells in circulation gain more benefits from Tα1. Moreover, Tα1 reduced PD-1 and Tim-3 expression on CD8+ T cells and increased T cell receptor excision circles, indicating it could reverse T cell exhaustion and recovers immune reconstitution through promoting thymus output during COVID-19 infection. However, in the present study we found the use of glucocorticoids or thymosin had no significant impact on outcome of COVID-19 patients. More studies are needed to further evaluate the application of Tα1 and other immunomodulatory drugs in the treatment of COVID-19 patients.
B cells and NK cells were also innate effector lymphocytes that responded to acute viral infections but might also contribute to immunopathology. In the present study, we found the absolute counts of B cells and NK cells were higher in the critical patients compared to other patients. A recent study analyzed circulating B cell subsets and their possible relationship with COVID-19 features and severity. It was found the severity of COVID-19 was accompanied by changes in the B cell sub-populations, either immature or terminally differentiated. 23 Another study had revealed strong NK cell activation across distinct subsets in peripheral blood of COVID-19 patients by using 28-color flow cytometry. Through unsupervised high-dimensional analysis of peripheral blood NK cells, they furthermore identified distinct NK cell immunotypes that were linked to disease severity. 24 Indepth investigation on these cells could help us better understand their roles during COVID-19.
There were also some limitations in our research. First, this was a single-center retrospective study, which needs to be verified by multi-center prospective studies. Second, some patients were admitted in hospital after a relatively long time from the onset of the disease, which could not accurately reflect the state of the initial infection. Third, this study lacked dynamic monitoring of lymphocyte subsets before and after treatment, especially lymphocyte subsets changes during the use of glucocorticoids or thymosin.
Conclusion
We analyzed blood cell counts and lymphocyte subsets in COVID-19 patients, and found their correlation with disease severity and outcome. Dynamic monitoring blood cell counts and lymphocyte subsets might be helpful to evaluate severity and guide treatment of the patients.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Science and Technology Plan Project of Guangzhou (201904010018, 202102021253), Rural Science and Technology Commissioner Plan Project of Guangdong Province (KTP20190256), Natural Science Foundation of Guangdong Province (2019A1515010978), Medical Scientific Research Foundation of Guangdong Province (B2021115), National Natural Science Foundation of China (81741133), Top Project for Military Medical Science and Technology Youth Training Program (21QNPY135).
Ethics approval: Ethical approval for this study was obtained from Ethnic Committee of Huoshenshan Hospital, Wuhan, China.
Informed consent: Written informed consent was waived by the Ethnic Committee of Huoshenshan Hospital, Wuhan, China.
ORCID iD
Peng Zhang https://orcid.org/0000-0001-7626-1977
References
- 1.Sahu KK, Mishra AK, Lal A. (2020) COVID-2019: update on epidemiology, disease spread and management. Monaldi Arch Chest Dis 90: 1292. [DOI] [PubMed] [Google Scholar]
- 2.Richardson S, Hirsch JS, Narasimhan M, et al. (2020) Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. J Am Med Assoc 323: 2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li R, Tian J, Yang F, et al. (2020) Clinical characteristics of 225 patients with COVID-19 in a tertiary Hospital near Wuhan, China. J Clin Virol 127: 104363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaira LA, Deiana G, Fois AG, et al. (2020) Objective evaluation of anosmia and ageusia in COVID-19 patients: Single-center experience on 72 cases. Head Neck 42: 1252–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pascarella G, Strumia A, Piliego C, et al. (2020) COVID-19 diagnosis and management: a comprehensive review. J Intern Med 288: 192–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen P, Zhou B. (2020) Clinical characteristics of COVID-19 patients with abnormal liver tests. J Hepatol 73: 712–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan BE, Chong V, Chan S, et al. (2020) Hematologic parameters in patients with COVID-19 infection. Am J Hematol 95: E131–E134. [DOI] [PubMed] [Google Scholar]
- 8.Xu Z, Shi L, Wang Y, et al. (2020) Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8: 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C, Wang Y, Li X, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. (2020) Hematological findings and complications of COVID-19. Am J Hematol 95(7): 834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang AP, Liu JP, Tao WQ, et al. (2020) The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol 84: 106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Vito A, Geremia N, Fiore V, et al. (2020) Clinical features, laboratory findings and predictors of death in hospitalized patients with COVID-19 in Sardinia, Italy. Eur Rev Med Pharmacol Sci 24: 7861–7868. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z, Long W, Tu M, et al. (2020) Lymphocyte subset (CD4+, CD8+) counts reflect the severity of infection and predict the clinical outcomes in patients with COVID-19. J Infect 81: 318–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan S, Yi Q, Fan S, et al. (2020) Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID-19) infected patients. Br J Haematol 189: 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao X. (2020) COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol 20: 269–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hajian-Tilaki K. (2014) Sample size estimation in diagnostic test studies of biomedical informatics. J Biomed Inform 48: 193–204. [DOI] [PubMed] [Google Scholar]
- 17.Li G, Fan Y, Lai Y, et al. (2020) Coronavirus infections and immune responses. J Med Virol 92: 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin C, Zhou L, Hu Z, et al. (2020) Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis 71: 762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan M, Liu Y, Zhou R, et al. (2020) Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology 160: 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu B, Fan CY, Wang AL, et al. (2020) Suppressed T cell-mediated immunity in patients with COVID-19: a clinical retrospective study in Wuhan, China. J Infect 81: e51–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pei F, Guan X, Wu J. (2018) Thymosin alpha 1 treatment for patients with sepsis. Expert Opin Biol Ther 18: 71–76. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Pang Y, Hu Z, et al. (2020) Thymosin alpha 1 (Tα1) reduces the mortality of severe COVID-19 by restoration of lymphocytopenia and reversion of exhausted T cells. Clin Infect Dis 71: 2150–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sosa-Hernández VA, Torres-Ruíz J, Cervantes-Díaz R, et al. (2020) B cell subsets as severity-associated signatures in COVID-19 patients. Front Immunol 11: 611004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maucourant C, Filipovic I, Ponzetta A, et al. (2020) Natural killer cell immunotypes related to COVID-19 disease severity. Sci Immunol 5(50): eabd6832. [DOI] [PMC free article] [PubMed] [Google Scholar]