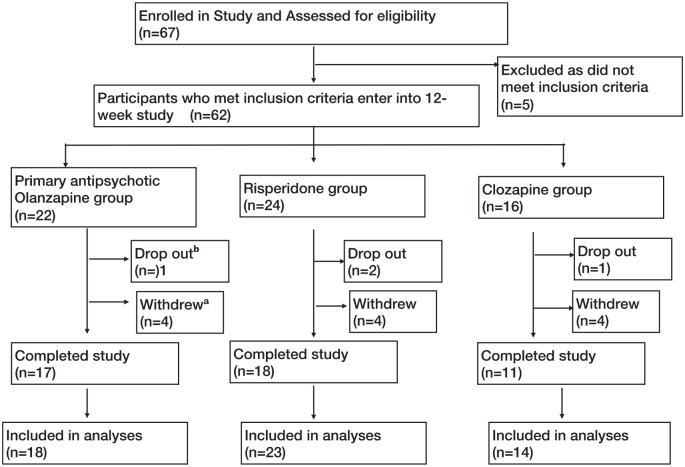
Figure 1.
Patient flow diagram through the trial as per CONSORT guidelines.
aSubjects were withdrawn from the study for any of the following reasons: non-compliance (n = 1), abnormal baseline blood results/abnormalities in the results such that medication changes were required (n = 4), social issues (n = 2), patient/family’s request (n = 4) and mental state deterioration (n = 1).
bSubjects dropped out from the study for any of the following reasons: loss to follow-up (n = 1) and side effects (n = 3).