Fig. 3. Integration of the circadian rhythms and feeding-fasting cycles with metabolism.
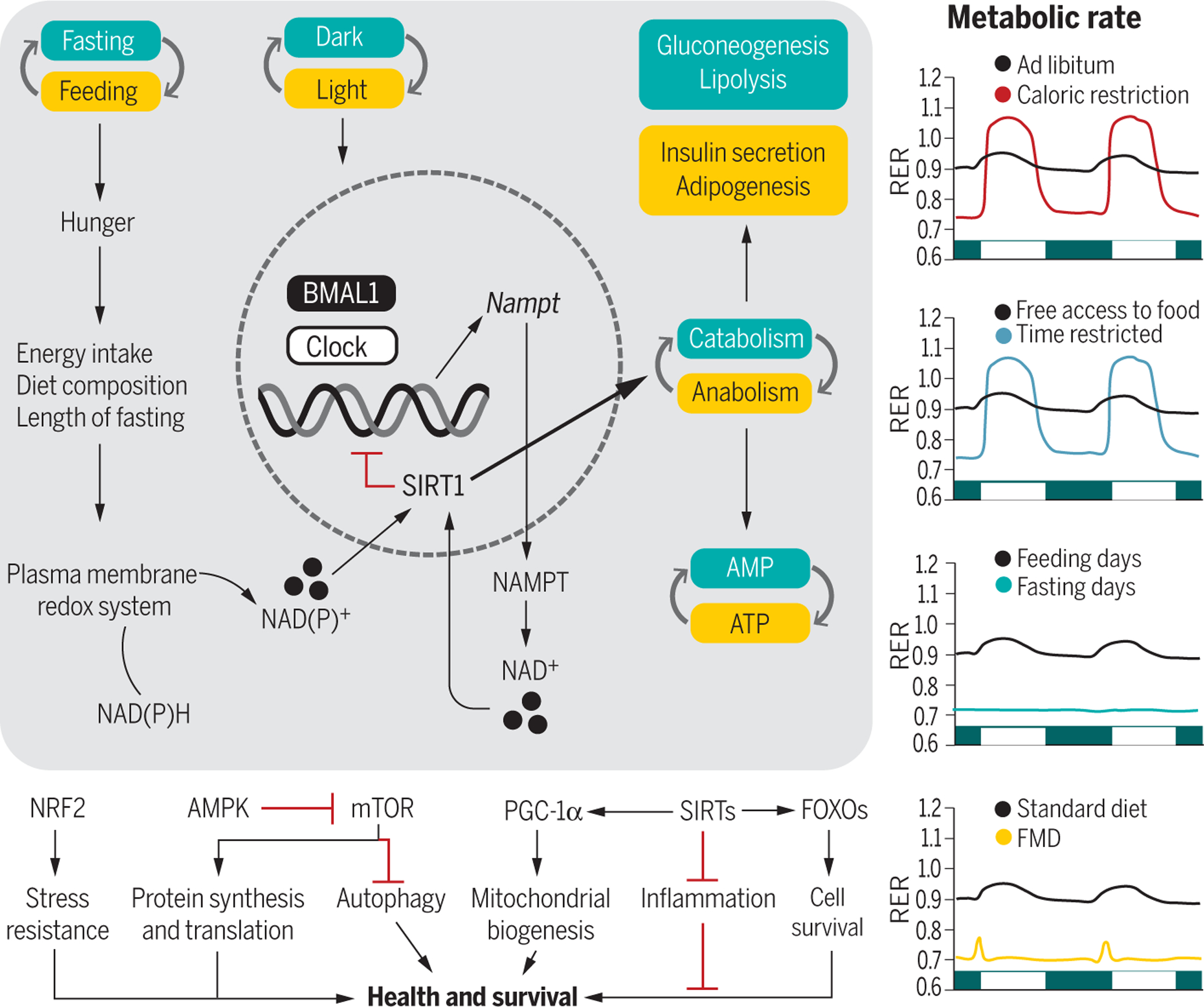
(Left) The transcriptional activators BMAL1 and CLOCK are at the core of a cell-autonomous molecular circuit that governs circadian rhythms. Fasting increases hunger, the extent of which depends on the overall energy intake, diet composition, and length of fasting. The internal circadian clock also increases hunger independent of food intake and other behaviors. Intermittent energy restriction increases concentrations of the plasma membrane redox system enzymes, NADH-cytochrome b5 reductase and NAD(P)H-quinone oxidoreductase, contributing to oscillations in the NAD(P)H [reduced form of NAD(P)+] to NAD(P)+ (nicotinamide adenine dinucleotide phosphate) ratio (82). The circadian rhythmicity of CLOCK and BMAL1 expression regulates the transcription of NAMPT, a key regulatory enzyme involved in the generation of NAD+, a metabolite required for the deacetylase activity of SIRT1. Active SIRT1 influences metabolism through its effects on catabolic and anabolic reactions and mediates BMAL1 deacetylation, which inhibits the circadian clock machinery. During the active phase (yellow boxes), increased production of ATP sustains anabolic pathways. During the resting phase (green boxes), a shift toward AMP gears metabolism toward catabolic processes. These intermediate energy carriers activate downstream transcription factors, kinases, and deacetylases like NRF2, AMPK, PGC-1α, sirtuins, and FOXOs, whose activation influences health and survival. (Right) At the organismal level, fasting or feeding states are paralleled by changes in the metabolic rate. AL–fed animals set their RERs at around 0.9, showing an intermediate preference between fat and carbohydrate metabolism. Both CR and TRF regimens increase the amplitude of RER oscillations, characterized by higher RER (utilization of carbohydrates) during feeding and lower RER (utilization of lipids) during fasting. Under prolonged fasting, lipids are the only source of energy, as opposed to feeding time. The FMD diet results in lower RER with a slight peak after the meal. The RER traces are idealized and may be close to what is seen in nocturnal rodents.
