TO THE EDITOR:
Covid-19 has shown to affect patients with hematological malignancies (HMs) more severely than the general population, with an estimated mortality rate of 33–37% [1–3]. In these patients, the rate of antibody response against SARS-CoV-2 infection has been estimated to be 69% [4].
At present, the anti-SARS-CoV-2 vaccination represents the most effective strategy for the prevention of Covid-19 in the general population. The Italian national vaccination plan included patients with HMs among the high-priority group and recommended the use of mRNA vaccines in this subset of patients [5]. The immunological response to anti-SARS-CoV-2 vaccines in patients with HMs is heterogeneous [6–12].
We promoted a monocentric, prospective, cohort study registered at ClinicalTrials.gov (identifier, NCT04878822), aimed to evaluate both humoral and cellular immune response to anti-SARS-CoV-2 vaccination in adult patients with HMs. Here, we report the results obtained in a subset of patients who underwent autologous stem cell transplantation (ASCT) as part of their HM treatment.
Methods
The cohort of the study included consecutive patients with HMs treated with ASCT at our Department of Hematology who had received anti-SARS-CoV-2 vaccination. Information was collected regarding patient demographics, HM characteristics, last HM treatment, ASCT, previous Covid-19. Post-vaccination cases of Covid-19 were detected through regular follow-up. We evaluated antibody and T-cell responses four weeks after the completion of the vaccination regimen. To test humoral immunity, we used the DiaSorin’s Liaison SARS-CoV-2 S1/S2 IgG test. All results were expressed as WHO international standard unit BAU/ml. A cutoff of anti-SARS-CoV2 IgG antibodies (Abs) >33.8 BAU/ml was considered as a positive test result. Cell-mediated immunity was tested in all seronegative patients and in an equal number of randomly selected seropositive patients (50% with high Ab titer and 50% with low Ab titer). The anti-spike T-cell-mediated immune response was tested by multicolor fluorescence-activated cell sorting (FACS) and enzyme-linked immunosorbent spot (ELISpot) assay. Detailed methods are described in Supplemental Information.
Univariate logistic regression model was used to assess the association between negative serology testing and baseline characteristics such as age, sex, HM type, HM status, absolute lymphocyte count (ALC), status of therapy, and type of last therapy. Odds ratios (OR) and 95% confidence intervals (CI) were calculated. The parameters with α = 0.10 were tested in a multivariate logistic regression with stepwise selection to assess the independent risk factors for vaccination failure. Antibody titer was summarized using geometric mean concentration (GMC) together with 95% CI. The Pearson correlation coefficient was used to evaluate the relationship between pre-vaccination ALC and GMC. To explore differences between GMC and type of last therapy we applied the ANOVA model. Regarding cell-mediated immunity, we used an unpaired two-tailed t-test to compare FACS results between the seronegative and seropositive groups. All other statistics were descriptive. Statistical analysis was performed with SAS version 9.4, Cary, NC, USA.
Results
At data cutoff (May 31, 2021), 64 patients who had undergone ASCT as part of their HM treatment entered the analysis: 32 (50%) had ASCT as their last treatment before vaccination (ASCT-LT), while the remaining did not (No-ASCT-LT). All patients received BNT162b2 (Comirnaty) vaccine.
Immunogenicity of anti-SARS-CoV-2 vaccine was tested at a median time of 28 days (range, 25–48) after the second dose of vaccine. Patients’ characteristics at the time of vaccine administration are reported in Supplemental Table ST1. Among the No-ASCT-LT cohort, 26 patients (81%) were undergoing treatment at the time of vaccination (Supplemental Table ST2).
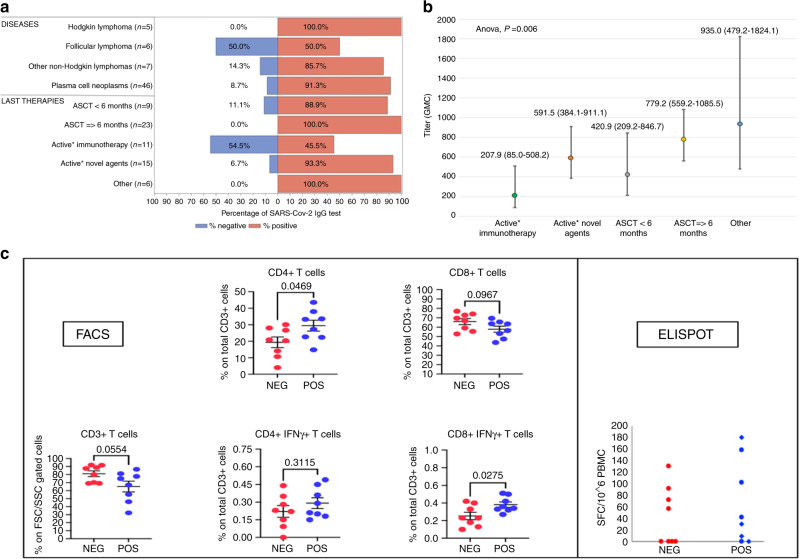
Overall, 56 out of 64 patients (87%) developed a humoral immune response, with a median Ab titer of 747 BAU/ml (range, 101–2018). Conversely, eight patients (13%) did not show detectable levels of Abs: one out of 32 patients (3%) in the ASCT-LT cohort and seven out of 32 (21%) in the No-ASCT-LT cohort (Fig. 1a).
Fig. 1. Data on humoral and cellular responses after anti-SARS-CoV-2 vaccine in 64 patients with hematological malignancies who underwent autologous stem cell transplantation.
a Seroconversion rates according to type of disease and type of last therapy. b Association between Ab titer’s GMC and type of last therapy. c FACS and ELISpot analyses of spike-specific T-cell responses according to serological status. ASCT: autologous stem cell transplantation; GMC: geometric mean concentration; NEG: seronegative after vaccination; POS: seropositive after vaccination; FSC/SSC: forward scatter/side scatter; SFC: spot-forming cells; PBMC: peripheral blood mononuclear cells. Immunotherapy includes monoclonal anti-CD38, monoclonal anti-CD20, and bispecific antibodies. Novel agents include immunomodulatory drugs and proteasome inhibitors. Other includes allogeneic stem cell transplantation, chemotherapy, and supportive therapy. *Active therapy was defined as ongoing therapy or therapy discontinued within 6 months from vaccination. In the ELISpot figure: blue rhombi: seropositive patients with low Ab titer; blue circles: seropositive patients with high Ab titer.
Among the ASCT-LT subgroup, the median time between ASCT and vaccination was 17.6 months (range, 1.2–58.1). Most patients (23 out of 32, 72%) were vaccinated at least 6 months after ASCT, however, all four patients vaccinated within 3 months from ASCT elicited a humoral immune response. In the No-ASCT-LT subpopulation, all patients without detectable humoral responses were on active therapy at the time of vaccination (Supplemental Table ST2).
The univariate analysis showed that not having ASCT as last treatment (OR 9.33, 95% CI 1.08–81.0; P = 0.04) and being on treatment (OR 12.60, 95% CI 1.45–109.82, P = 0.02) were significantly associated with vaccination failure. The effect of being on treatment remained significant in the multivariate analysis (OR 49.45, 95% CI 2.88–849.62; P = 0.007). Concerning the role of ALC, we found no association with seroconversion failure (P = 0.40), but a positive correlation with Ab titer’s GMC (ϱ = 0.48, P = 0.0008). Finally, the ANOVA model disclosed a significant difference between Ab titer’s GMC and type of last therapy (F test P = 0.006) (Fig. 1b).
Regarding cell-mediated immunity, the FACS analysis showed a statistically significant lower percentage of spike-specific CD8+IFNγ+ T-cells (P = 0.027) and CD4+ T-cells (P = 0.047) in the seronegative group as compared to the seropositive group. No difference between the two groups was found in terms of spike-specific CD3+ T-cells, CD8+ T-cells, and CD4+IFNγ+ T-cells. The ELISpot assay showed a lower percentage of spike-specific IFNγ-producing T-cells in seronegative patients compared to seropositive patients (50% vs. 75%). Among all responders, we detected four patients with a high magnitude response (arbitrarily defined as >100 spot-forming cells/106 peripheral blood mononuclear cells), one of them (12.5%) had a negative humoral response (Fig. 1c and Supplemental Table ST3).
Concerning post-vaccination Covid-19, we had one case of severe disease requiring hospitalization, caused by the Alpha variant of SARS-CoV-2. The patient was a 56-year-old male affected by multiple myeloma in partial response to Dara-RD treatment, who had undergone ASCT 57 months before vaccination. The first Comirnaty dose was administered 10 days after his last Dara infusion. The onset of symptoms occurred 26 days after the second vaccine dose. The serological response was evaluated during admission and resulted positive (543 BAU/ml).
Discussion
Overall, in 64 ASCT recipients the rate of seroconversion was 87%. These results are encouraging when compared to those reported in other subsets of patients with HMs mainly assessed after a single vaccine dose [6–11]. In our study, patients who underwent ASCT as last treatment largely seroconverted, even when vaccinated within 3 months from ASCT. On the other hand, the study showed a significant association between ongoing HM treatment and lower rate of post-vaccination seroconversion. Immunotherapy was associated with the highest risk of seroconversion failure. Furthermore, even in those who mounted a humoral response, immunotherapy appeared to negatively influence its magnitude. As evidence for this, the only patient who developed post-vaccination Covid-19 was on active Dara-RD treatment. These results are consistent with those reported by Maneikis et al. [12].
Concerning cellular immune response, we documented in vitro SARS-CoV-2-specific T-cell responses both in seropositive and seronegative patients, albeit at lower frequencies in the latter. Further studies are necessary to better understand the clinical implications of such findings and longer follow-up is needed to assess the degree of clinical protection conferred by vaccination.
This study has some limitations, such as the small sample size, the absence of matched healthy controls and of baseline immune evaluation. However, our data suggest that most ASCT recipients are likely to seroconvert if out of treatment, while this rate lowers when patients are on treatment, especially on immunotherapy. Assessing antigen-specific T-cell responses in patients with HMs is useful to better understand their immune profile.
Supplementary information
Acknowledgements
We thank Susanna Bassi from the Clinical Trial Center of Hematology at the A.S.S.T. Sette Laghi of Varese (Italy) serving as research nurse. We thank Nicole Fabbri for participating in patient enrollment.
Author contributions
F.P., M.S., A.P.G., F.M., L.M. and A.B. (A. Bruno) designed research and analyzed data; F.M. and A.B. (A. Baj) did the serological analysis; L.M. and R.B. performed the ELISpot analysis; M.G. performed the FACS staining and sample acquisition; A.B. (A. Bruno) and M.G. performed the FACS analysis; L.B. did the statistical analysis; M.S., F.P., C.D., L.M. and A.B. (A. Bruno) wrote the paper; C.D., B.M., M.B., R.M., A.I., D.S., B.B., S.A., D.B., M.M. and A.F. collected clinical data.
Funding Information
The study has been supported by the charity AIL (Associazione italiana contro le leucemie-linfomi e mieloma) Onlus of Varese, by grants from the Ministero della Salute, Rome, Italy (Finalizzata 2018, NET-2018-12365935, Personalized medicine program on myeloid neoplasms: characterization of the patient’s genome for clinical decision making and systematic collection of real world data to improve quality of health care), by grants from the Ministero dell’Istruzione, dell’Università e della Ricerca, Roma, Italy (PRIN 2017, 2017WXR7ZT; Myeloid neoplasms: an integrated clinical, molecular and therapeutic approach) and by grants from Fondazione Matarelli, Milan. A.B. (A. Bruno) has received funds from the Italian Association for Cancer Research (AIRC-MFAG, ID-22818 and the Cariplo Foundation (ID-2019-1609). A.B. (A. Bruno) is supported by the Italian Ministry of Health Ricerca Corrente-IRCCS MultiMedica. M.G. is a participant of PhD course in Life Sciences and Biotechnology at the University of Insubria and is funded by an “assegno di ricerca”.
Competing interests
The authors declare no competing interests.
Ethics approval
The study was approved by the institutional review board ethics committees (protocol n. 84-2021) and conducted in accordance with the Helsinki Declaration of 1975 as revised in 2013. All participants signed a written informed consent.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41409-021-01487-4.
References
- 1.Passamonti F, Cattaneo C, Arcaini L, Bruna R, Cavo M, Merli F, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7:737–45. doi: 10.1016/S2352-3026(20)30251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vijenthira A, Gong IY, Fox TA, Booth S, Cook G, Fattizzo B, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136:2881–92. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.García-Suárez J, de la Cruz J, Cedillo Á, Llamas P, Duarte R, Jiménez-Yuste V, et al. Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: lessons from a large population-based registry study. J Hematol Oncol. 2020;13:133. doi: 10.1186/s13045-020-00970-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Passamonti F, Romano A, Salvini M, Merli F, Della Porta MG, Bruna R, et al. COVID-19 elicits an impaired antibody response against SARS-CoV-2 in patients with haematological malignancies. Br J Haematol. 2021. 10.1111/bjh.17704. [DOI] [PMC free article] [PubMed]
- 5.Vaccinazione anti-SARS-CoV-2/COVID-19. Raccomandazioni ad interim sui gruppi target della vaccinazione anti-SARS-CoV-2/COVID-19. VaccinarSì. 2021; https://www.trovanorme.salute.gov.it/norme/renderPdf.spring?seriegu=SG&datagu=24/03/2021&redaz=21A01802&artp=1&art=1&subart=1&subart1=10&vers=1&prog=002.
- 6.Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137:3165–73. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roeker LE, Knorr DA, Thompson MC, Nivar M, Lebowitz S, Peters N, et al. COVID-19 vaccine efficacy in patients with chronic lymphocytic leukemia. Leukemia. 2021;35:2703–5. doi: 10.1038/s41375-021-01270-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terpos E, Trougakos IP, Gavriatopoulou M, Papassotiriou I, Sklirou AD, Ntanasis-Stathopoulos I, et al. Low neutralizing antibody responses against SARS-CoV-2 in older patients with myeloma after the first BNT162b2 vaccine dose. Blood. 2021;137:3674–6. doi: 10.1182/blood.2021011904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bird S, Panopoulou A, Shea RL, Tsui M, Saso R, Sud A, et al. Response to first vaccination against SARS-CoV-2 in patients with multiple myeloma. Lancet Haematol. 2021;8:389–92. doi: 10.1016/S2352-3026(21)00110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Oekelen O, Gleason CR, Agte S, Srivastava K, Beach KF, Aleman A, et al. Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple myeloma. Cancer Cell. 2021;39:1028–30. doi: 10.1016/j.ccell.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monin L, Laing AG, Muñoz-Ruiz M, McKenzie DR, Del Molino Del Barrio I, Alaguthurai T, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–78. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maneikis K, Šablauskas K, Ringelevičiūtė U, Vaitekėnaitė V, Čekauskienė R, Kryžauskaitė L, et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. Lancet Haematol. 2021;8:e583–92. doi: 10.1016/S2352-3026(21)00169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.