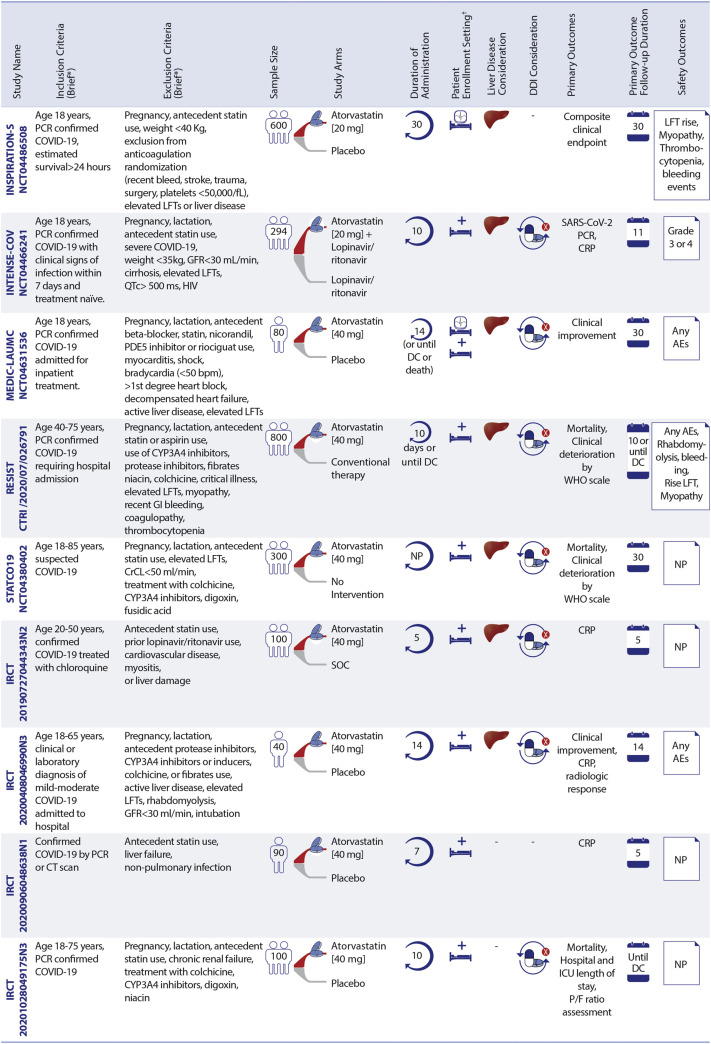
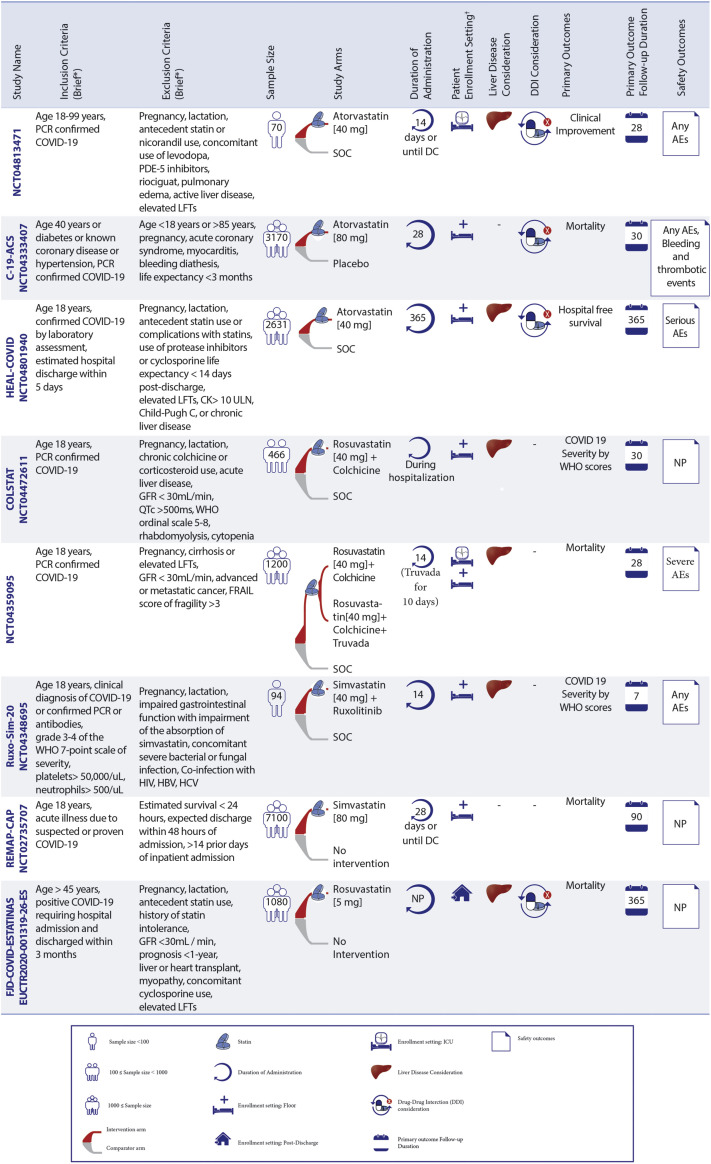
Figure 3.
Ongoing Statin Therapy RCTs in Patients With COVID-19
Statin trials are evaluating patients in different settings. ∗See full list of inclusion/exclusion criteria in the original trial records. †Enrollment setting includes hospitalized and post-discharge. AEs = adverse events; CK = creatine kinase; CrCl = creatinine clearance; CRP = C-reactive protein; CT = computed tomography; DC = discharge; DDI = drug–drug interaction; FRAIL = Fatigue Resistance, Ambulation, Illnesses, and Loss of weight questionnaire; GFR = glomerular filtration rate; HBV = hepatitis B virus; HCV = hepatitis C virus; LFT = liver function tests; MI = myocardial ischemia; NP = not pointed; P/F ratio = arterial oxygen partial pressure/fractional inspired oxygen; PDE5 = phosphodiesterase type 5; SOC = standard of care; RCTs = randomized controlled trials; C-19-ACS = Preventing cardiac complications of COVID-19 disease with early acute coronary syndrome therapy: a randomized controlled trial; COLSTAT = Colchicine/Statins for the prevention of COVID-19 complications; RESIST = A randomized control trial of statin and aspirin as adjuvant therapy in patients with SARS-CoV-2 infection; STATCO19 = Atorvastatin as adjuvant therapy in COVID-19. All other full study names are provided in the text.