FIGURE 8.
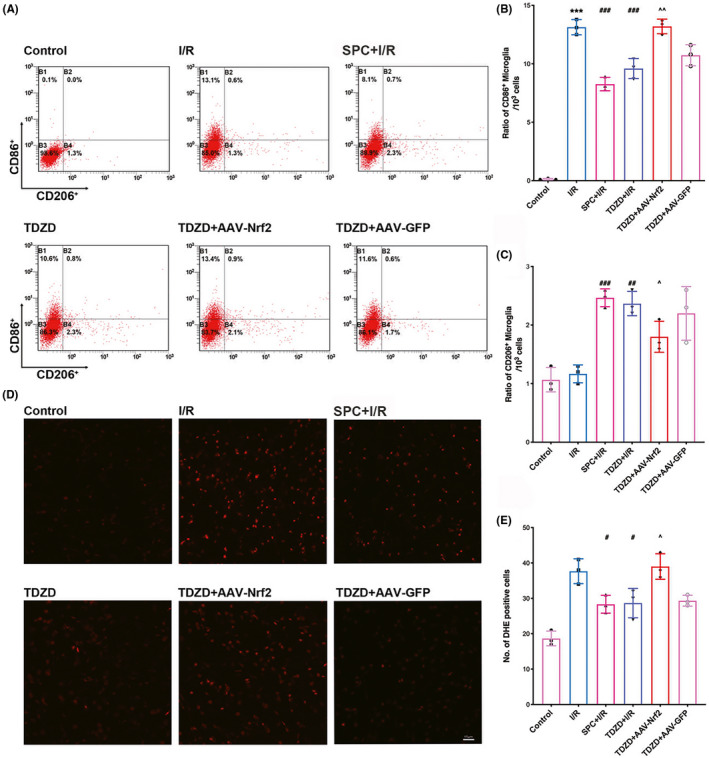
SPC and TDZD treatment alleviated pro‐inflammatory microglial but increased anti‐inflammatory microglial phenotype polarization and reduced the generation of ROS. These effects were reversed by the deficiency of Nrf2. (A) Flow cytometry analysis of CD86+ (pro‐inflammatory microglia) and CD206+ (anti‐inflammatory) cells in the ischemic penumbra 7 days after reperfusion. (B, C) The quantitative statistical results of CD206‐positive cells (B) and CD86‐ (C) in each group (n = 3). (D) Representative immunofluorescence micrographs showing DHE staining for ROS in each group. Scale bar = 15 μm. (E) The quantitative analysis of DHE‐positive cells. *p < 0.05, **p < 0.01, ***p < 0.001 versus the I/R group; #p < 0.05, ##p < 0.01, ###p < 0.001 versus the I/R group; ^p < 0.05, ^^p < 0.01, ^^^p < 0.001. One‐way ANOVA with Tukey's post hoc test was used for statistical analysis. AAV‐Nrf2, adeno‐associated virus induced Nrf2‐shRNA; DHE, dihydroethidium; I/R, ischemia/reperfusion; Sevo, sevoflurane preconditioning; TDZD, 4‐Benzyl‐2‐methyl‐1,2,4‐thiadiazolidine‐3,5‐dione
