Abstract
Phenylketonuria (PKU), a deficiency in the activity of the enzyme phenylalanine hydroxylase, leads to toxic levels of phenylalanine (Phe) in the blood and brain. Pegvaliase (recombinant Anabaena variabilis phenylalanine ammonia lyase conjugated with polyethylene glycol) is approved to manage PKU in patients aged greater than or equal to 18 years in the United States and in patients aged greater than or equal to 16 years in the European Union. Pharmacokinetic, pharmacodynamic, and immunogenicity results from five open‐label pegvaliase trials were assessed. Studies with induction/titration/maintenance (I/T/M) dosing regimens demonstrated pharmacokinetic stabilization and sustained efficacy associated with maintenance doses (20, 40, or 60 mg/day). Immune‐mediated pegvaliase clearance was high during induction/titration phases when the early immune response was peaking. The combination of low drug dosage and high drug clearance led to low drug exposure and minimal decreases in blood Phe levels during induction/titration. Higher drug exposure and substantial reductions in blood Phe levels were observed later in treatment as drug clearance was reduced due to the maturation of the immune response, which allowed for increased dosing to target levels. The incidence of hypersensitivity reactions was temporally associated with the peaking of the early antidrug immune response and decreased with time as immune response matured after the first 6 months of treatment. These results support an I/T/M dosing regimen and suggest a strategy for administration of other nonhuman biologics to achieve efficacy and improve tolerability.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Immunogenicity is a challenge for treatment with biologic therapeutics, particularly with enzymes of nonhuman origin. Pegvaliase is approved to treat phenylketonuria in adults and patients aged greater than or equal to 18 years in the United States and aged greater than or equal to 16 years in the European Union and is the first bacterially derived protein approved for treatment of a chronic disease.
WHAT QUESTION DID THIS STUDY ADDRESS?
To improve pharmacodynamic stability and reduce immune clearance of the bacterially derived phenylalanine ammonia lyase (PAL), the formulation of pegvaliase includes conjugation of PAL with polyethylene glycol (PEG). However, previous studies of PEGylated drugs have described the development of anti‐PEG antibodies associated with reduced efficacy and hypersensitivity reactions. This study investigated the use of an induction/titration/maintenance (I/T/M) dosing regimen to minimize the immune response expected with pegvaliase due to the inclusion of PEG and bacterially derived PAL.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Immunogenicity plays the most salient role in pegvaliase pharmacokinetics, primarily via drug clearance during early treatment. The introduction of an I/T/M dosing regimen allowed for sustained efficacy with acceptable tolerability.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
This dosing strategy may provide a method for the administration of other biologic drugs that are of nonhuman origin, including PEGylated drugs.
INTRODUCTION
Phenylketonuria (PKU; OMIM 261600) is an autosomal recessive disorder characterized by a deficiency in the enzyme phenylalanine hydroxylase (PAH), which converts phenylalanine (Phe) to tyrosine. 1 In this disorder, toxic levels of Phe accumulate in the blood and brain, resulting in negative effects on mood, anxiety, behavior, and executive function. 2 , 3 Treatment guidelines from the American College of Medical Genetics and Genomics recommend targeting blood Phe levels below 360 µmol/L because blood Phe levels closer to normal levels (mean normal level: 60 μmol/L) can prevent or improve neuropsychological symptoms. 1 , 2
The key management strategy for PKU—severe dietary restriction of Phe through decreased intake of natural protein and the use of low‐Phe medical formulas—is challenging long‐term because most foods contain Phe. Approximately 30% of children and adolescents and 75% of adults are unable to maintain blood Phe levels within the recommended range. 4 , 5 Enzyme replacement therapy is not an option for PKU because PAH is a liver enzyme that is unstable in the bloodstream. Patients with residual PAH activity may respond to treatment with sapropterin dihydrochloride (KUVAN; BioMarin Pharmaceutical Inc.), 6 a synthetic form of the PAH cofactor, tetrahydrobiopterin (BH4). 1 However, sapropterin is effective in only ~ 20%–56% of patients with PKU. 1 , 7 Therefore, novel therapies that do not rely on the existing enzymatic activity of PAH are needed for patients unable to maintain Phe levels within the recommended range.
Pegvaliase (PALYNZIQ; BioMarin Pharmaceutical Inc.), an enzyme substitution therapy, is a bacterially derived (recombinant Anabaena variabilis) Phe ammonia lyase (PAL) produced in Escherichia coli and conjugated with polyethylene glycol (PEG). 8 Unlike PAH, which requires BH4 to metabolize Phe, pegvaliase does not require a cofactor to convert Phe to ammonia and trans‐cinnamic acid, which is converted to hippuric acid and excreted in urine. 9 Pegvaliase is approved for managing PKU in the United States for patients aged greater than or equal to 18 years and in the European Union for patients aged greater than or equal to 16 years, and is the first bacterially derived therapeutic protein approved for treating a chronic disease. 10 , 11
Immunogenicity is a common concern with biologic therapeutics, particularly with enzymes of nonhuman origin. Antidrug antibody (ADA) immune complexes can form as a result of the immune response to the drug, modulating the drug’s pharmacokinetics (PKs) and/or pharmacodynamics (PDs). This may result in altered efficacy and an increased risk of hypersensitivity adverse events (HAEs). 12 , 13 , 14 , 15
The PEGylation approach has been used to improve PD stability and reduce immunogenicity‐related blood clearance for many biologic therapeutics, including nonhuman‐origin enzymes, such as asparaginase, methioninase, and lysostaphin. 16 , 17 , 18 Previous studies of PEGylated drugs have described the development of anti‐PEG antibodies (Abs), which were associated with reduced efficacy and hypersensitivity reactions. 19 , 20 An immunogenic response was therefore expected with pegvaliase due to the inclusion of bacterially derived PAL.
PK data from an open‐label, single‐dose, dose‐escalation phase I trial of pegvaliase in patients with PKU (PAL‐001; NCT00634660) demonstrate dose‐dependent peak drug concentrations at 89–106 h and elimination half‐lives of 46–120 h. 19 These findings supported once‐weekly dosing in the first phase II trials. However, subsequent phase II and III efficacy, safety, and immunogenicity data (NCT00924703, NCT01560286, NCT00925054, NCT01212744, NCT01819727, and NCT01889862) supported more frequent dosing with the inclusion of an induction/titration/maintenance (I/T/M) dosing regimen that led to substantial reductions in blood Phe levels with a manageable safety profile for most subjects. 19 , 21 , 22 , 23 , 24 , 25 , 26
Herein, we describe the PK and PD following multiple doses of pegvaliase and the effects of immunogenicity in determining a safe, tolerable, and efficacious long‐term dosing regimen.
METHODS
Study design and patients
The methods for studies reported herein were previously published. 19 , 21 , 22 , 23 , 24 , 25 , 26 In brief, PAL‐002 was an open‐label, multicenter, single‐arm phase II study in which pegvaliase was administered via subcutaneous injection in weekly doses of 0.001–0.1 mg/kg/day over an 8‐week induction phase followed by an 8‐week titration phase. 19 , 23 In the open‐label, multicenter phase II PAL‐004 trial, daily doses of 0.06–0.8 mg/kg/day were administered for 13 weeks. 23 PAL‐165–205 was an open‐label, multicenter phase II study in which patients received pegvaliase 2.5 mg/week for 4–8 weeks and were titrated to a maximum of 75 mg/day over 24 weeks. 24 The phase III PRISM‐1 trial was an open‐label, multicenter, parallel‐group study in patients randomized 1:1 to receive a 20 or 40 mg/day maintenance dose of pegvaliase. All patients first received pegvaliase 2.5 mg/day for 4 weeks, which was titrated to randomized doses. 22 The maintenance phase, during which patients received 20 or 40 mg/day, lasted 24–36 weeks. Patients who reached a maintenance dose in PRISM‐1 were transitioned to part 1 of PRISM‐2, a subsequent open‐label, multicenter, 4‐part phase III study. In PRISM‐2, patients continued the same dose as in PRISM‐1 (part 1; 13 weeks). Patients who had achieved at least a 20% decrease from baseline in blood Phe levels were then randomized to continue their dose or receive placebo (part 2; 8 weeks). In part 3 (6 weeks), they received their original maintenance dose. Pegvaliase was suspended during week 6 of part 3 to allow characterization of elimination. In part 4, a dose of 5–60 mg/day was adjusted based on tolerability. All studies initially enrolled patients aged greater than or equal to 16 years (after recruitment began, protocols were amended to enroll patients aged ≥18 years) who were diagnosed with PKU and had a blood Phe concentration greater than 600 µmol/L at screening and for greater than or equal to 6 months before the study.
Institutional review boards of all participating institutions approved the study protocols. All procedures were done in accordance with the Guideline for Good Clinical Practice as issued by the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use. All patients provided written informed consent.
PK assay
Pegvaliase was measured in human plasma using quantitative sandwich enzyme‐linked immunosorbent assay (ELISA) methods. To reduce interference from ADAs due to competition with assay capture and detection reagents, samples were acidified and neutralized to dissociate ADAs from pegvaliase before addition to the assay plate. For phase II studies, the sandwich ELISA consisted of a rAvPAL‐PEG IgG capture antibody and a rabbit monoclonal anti‐PEG IgG detection antibody (lower limit of quantitation [LLOQ]: 4 ng/ml in neat plasma; Table S1). Phase III studies used the rabbit monoclonal anti‐PEG IgG reagent to capture pegvaliase and the rabbit polyclonal anti–rAvPAL‐PEG IgG reagent for detection (LLOQ: 75 ng/ml in neat plasma). All studies used a biotinylated detection reagent, followed by streptavidin‐conjugated horseradish peroxidase and tetramethylbenzidine substrate for colorimetric development. The concentration of pegvaliase in study samples was determined by interpolating raw assay signals against the standard calibration curve.
PD assay
A quantitative ion‐exchange chromatography method to measure amino acids Phe and tyrosine in human plasma was validated at ARUP Laboratories (Salt Lake City, UT) to support clinical studies of pegvaliase. Amino acids in de‐proteinized plasma were injected into a high‐performance lithium cation exchange chromatography column and separated using a gradient of pH, ionic strength, and temperature. The amino acids eluting from the column were mixed with ninhydrin and passed through a high‐temperature reaction coil to form colored adducts. Light absorbance of these adducts was measured with a photometer unit at 570 nm and integrated as a series of peaks with a specific retention time for each amino acid, with the area of each peak proportional to the amino acid concentration. The concentration of each amino acid was calculated using an internal standard (L‐norleucine) to adjust for differences in extraction and/or injection.
Antibody assays
Immunogenicity assays were conducted as described previously. 21 , 24 Briefly, semiquantitative direct‐format ELISA methods were developed to monitor anti‐PAL IgG, anti‐PAL IgM, anti‐PEG IgG, and anti‐PEG IgM in phase I and II studies. These methods were later redeveloped into more sensitive electrochemiluminescence assays (ECLAs) and validated, along with a total anti‐pegvaliase antibody titer ECLA, to support phase III studies. Neutralizing antibody titers were measured in phase III studies using a semiquantitative hybrid ligand‐binding liquid chromatography/tandem mass spectrometry method. Complement‐activating circulating immune complexes containing IgG and complement component 3d (C3d) were measured in serum using the MicroVue CIC‐Raji Cell Replacement ELISA immunoassay (EIA; Quidel Corporation, San Diego, CA). The MicroVue assay was modified to measure circulating immune complexes containing IgM and C3d. Decreases in C3 and C4 levels were monitored in serum as biomarkers of complement pathway activation.
PK analysis
Intensive PK samples were taken at the following time points from all patients receiving 20 or 40 mg/day pegvaliase in part 3 (N = 57) of PRISM‐2: predose, 2, 4, 8, 12, and 24 h on day 7 of week 1; and predose, 2, 4, 8, 12, 24, 48, 72, 96, 144, and 168 h in weeks 5 and 6. PK parameters were calculated using standard noncompartmental analysis (NCA) according to current working practices and using Phoenix WinNonlin version 6.4 (Certara USA Inc.). Below the limit of quantifications (BLQs) were replaced with zero when they occurred before peak concentration time (Tmax) and treated as missing if they occurred after Tmax. NCA analysis was also conducted on intensive PK samples collected from patients in part 3 of PRISM‐2.
Analysis of the effects of dietary phenylalanine intake
Patients were asked to maintain consistent natural protein intake during PRISM‐1 and PRISM‐2, with an allowance to increase protein intake if Phe levels were below 30 µmol/L. The impact of dietary Phe intake on the PK/PD relationships between the pegvaliase trough plasma concentrations (C trough) and blood Phe level in PRISM‐1 and PRISM‐2 was assessed by plotting pegvaliase C trough against time‐matched blood Phe levels according to quartiles of dietary Phe intake measured on the same day or the day before PK and PD sampling. To assess the impact of dietary Phe intake on PD parameters, PK and PD data from PRISM‐1 and PRISM‐2 were fitted to an inhibitory sigmoid maximum drug effect (Imax) model using Phoenix WinNonlin version 6.4:
where E0 represents the blood Phe level when the plasma pegvaliase concentration is zero, Imax represents the maximum effect, IC50 represents the plasma concentration of pegvaliase required for obtaining 50% of the maximum drug effect, C pegvaliase represents the plasma pegvaliase concentration, and gamma (γ) represents the Hill coefficient.
Statistical analyses
Pegvaliase C trough were summarized descriptively. The impact of ADAs on pegvaliase C trough was assessed per treatment group. PK and PD end points included the relationship between plasma exposure of pegvaliase and efficacy (e.g., percent change and absolute change in blood Phe concentration from baseline) and safety end points of interest (e.g., occurrence of serious adverse events [SAEs], HAEs, anaphylaxis). Significance of the impact of dietary Phe intake on the PD parameter was tested with t‐test.
RESULTS
Patient characteristics
Demographics and baseline characteristics for patients enrolled in the pegvaliase clinical trials have been presented in detail elsewhere. 19 , 21 , 22 , 23 , 24 , 25 , 26 In brief, the 5 studies discussed herein enrolled a total of 337 patients (range per study: 16–261). The range of mean baseline ages was 26.1–32.2 years, body mass index was 26.5–29.5, and blood Phe concentration was 1178.7–1482.1 μmol/L. The majority of patients did not adhere to a restricted diet, and the mean dietary Phe intake at baseline was between 1355 and 1975 mg/day.
PK and PD following weekly and daily dosing
During the first 8 weeks of study PAL‐002 (NCT00925054), 40 patients received a weekly fixed dose of pegvaliase ranging from 0.001 mg/kg to 0.1 mg/kg. Pegvaliase concentrations were BLQ in most plasma samples, and, consequently, no clinically significant changes in blood Phe levels were observed (Figure 1a). Based on the limited efficacy observed in PAL‐002, 16 patients in study PAL‐004 (NCT01212744) received pegvaliase at higher starting doses (0.060 to 0.400 mg/kg), which were administered more frequently (5 days per week). A marked reduction occurred in mean ± SD blood Phe levels from baseline (1482.1 ± 363.5 μmol/L) to week 2 (553.0 ± 563.5 μmol/L), but levels were not sustained because associated HAEs led to pegvaliase dose reductions and/or interruptions (Figure 1b).
FIGURE 1.
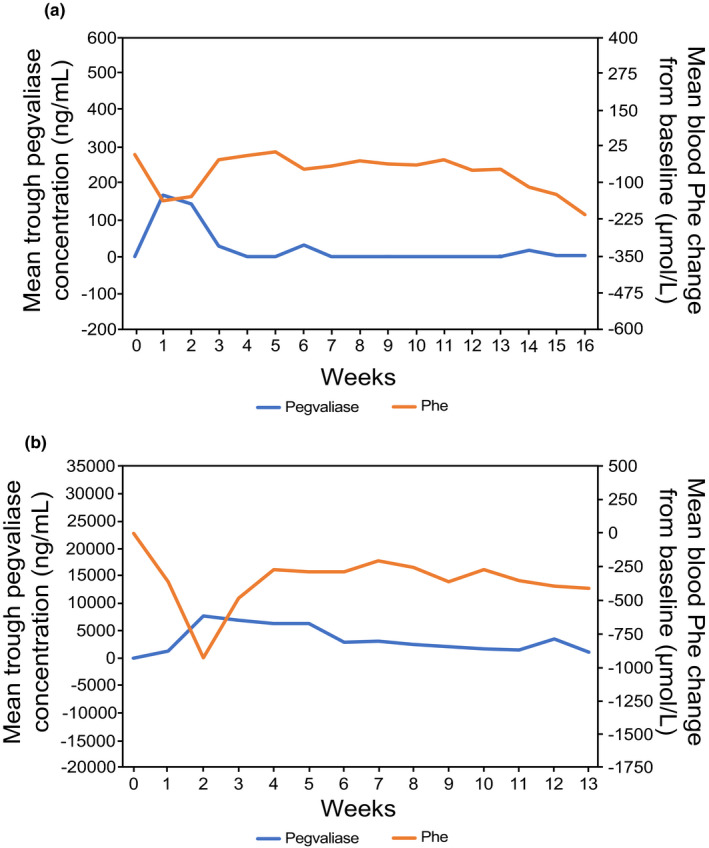
Mean ± standard deviation pegvaliase C trough and Phe concentrations over time. Subjects who discontinued prematurely were not replaced. (a) Study PAL‐002 (N = 40): subjects received a weekly fixed dose of pegvaliase ranging from 0.001 mg/kg to 0.1 mg/kg. (b) Study PAL‐004 (N = 16): subjects received a daily dose of pegvaliase ranging from 0.060 to 0.400 mg/kg (5 days per week). C trough, trough plasma concentration; Phe, phenylalanine; PKU, phenylketonuria
PK and PD with I/T/M dosing
An I/T/M dosing regimen for pegvaliase was first evaluated in the 24‐week phase II 165–205 trial (NCT01560286), in which sustained blood Phe level reduction was observed when maintenance doses were achieved. In this trial, 11 (46%) of the 24 patients achieved a maintenance dose (5–75 mg/day) within 24 weeks of initiating dosing that was sufficient to maintain blood Phe less than or equal to 600 μmol/L (Figure 2a). For those patients, plasma pegvaliase concentrations remained quantifiable and relatively stable throughout the study. Among 13 patients who did not achieve a maintenance dose within 24 weeks, 10 patients continued the treatment in the long‐term extension study when patients were allowed to titrate to higher doses to overcome immune‐mediated clearance and 8 of them reached blood Phe reduction. 24
FIGURE 2.
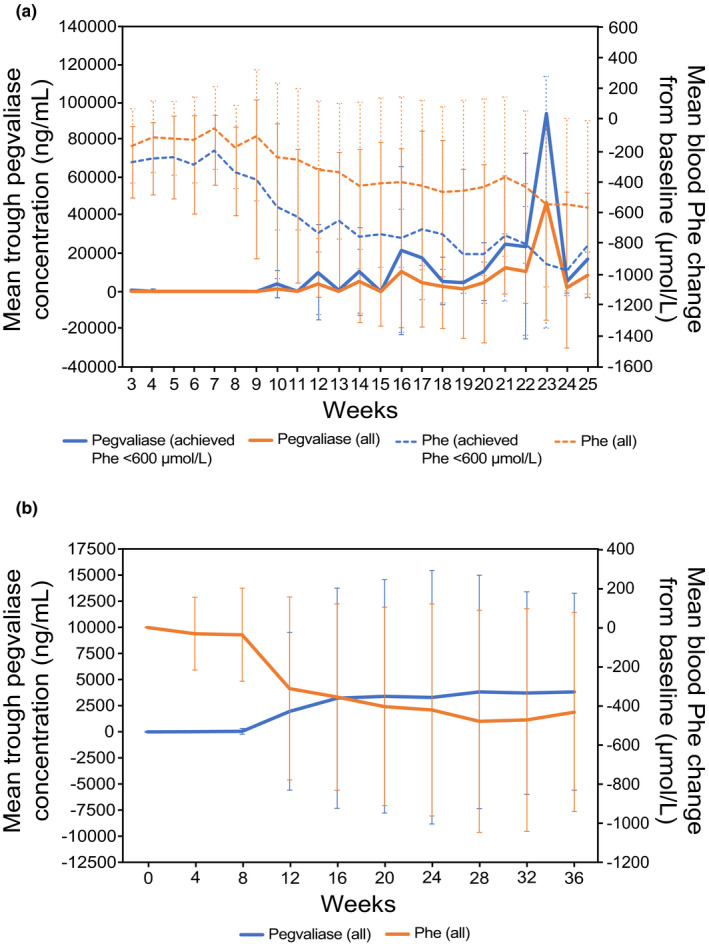
Mean ± SD pegvaliase C trough and Phe concentrations over time. (a) The 24‐week, open‐label phase II study PAL‐165–205 (N = 24) used an induction (2.5 mg/week) phase, followed by dose titration (5–75 mg/day) and maintenance dosing (dose at which patients sustained Phe ≤ 600 µmol/L) in adults with PKU. The figure shows pegvaliase and Phe levels among all patients (All, N = 24) and those who achieved Phe less than or equal to 600 µmol/L (achieved, N = 11). (b) PRISM‐1 was an open‐label, 36‐week phase III study (N = 261) in adults with PKU that used an induction phase (2.5 mg/week for 4 weeks), dose titration (randomized fixed dose of 20 or 40 mg/day for ≤30 weeks), and maintenance phase (≥3 weeks at the randomized dose). C trough, trough plasma concentration; Phe, phenylalanine; PK, pharmacokinetic; PKU, phenylketonuria
The I/T/M dosing regimen was further evaluated in the phase III PRISM‐1 (NCT01819727) and PRISM‐2 (NCT01889862) trials and led to substantial reduction in blood Phe levels and manageable tolerability for most subjects. 21 , 23 , 25 In PRISM‐1, patients were randomized 1:1 to titrate to a maintenance dose of 20 or 40 mg/day. Patients continuing to the PRISM‐2 long‐term extension could receive maintenance doses of pegvaliase up to 60 mg/day. An inverse correlation between plasma pegvaliase concentrations and blood Phe level reduction was observed in these studies. In PRISM‐1 (N = 261), the mean pegvaliase C trough was 61.3 ± 279.9 ng/ml at week 8, increased notably at week 12 to 1974.0 ± 7546.7 ng/ml, and reached steady‐state by week 24 (3310.6 ± 12,117.4 ng/ml; Figure 2b). This was associated with changes in blood Phe levels: 1232.7 ± 386.4 μmol/L at baseline, 1198.2 ± 402.4 μmol/L at week 8, 928.0 ± 527.5 μmol/L at week 12, and 799.5 ± 515.8 μmol/L at week 24. Similar correlation between plasma pegvaliase concentrations and blood Phe level reduction was also observed in PRISM‐2 (Figure 3). Overall, blood Phe reduction to less than or equal to 360 μmol/L was achieved by 60.7% (95% confidence interval [CI]: 54.4% to 67.1%) of subjects by 24 months, and 51.2% (95% CI: 44.8% to 58.0%) of subjects achieved a blood Phe concentration of less than or equal to 120 μmol/L by 24 months. 22
FIGURE 3.
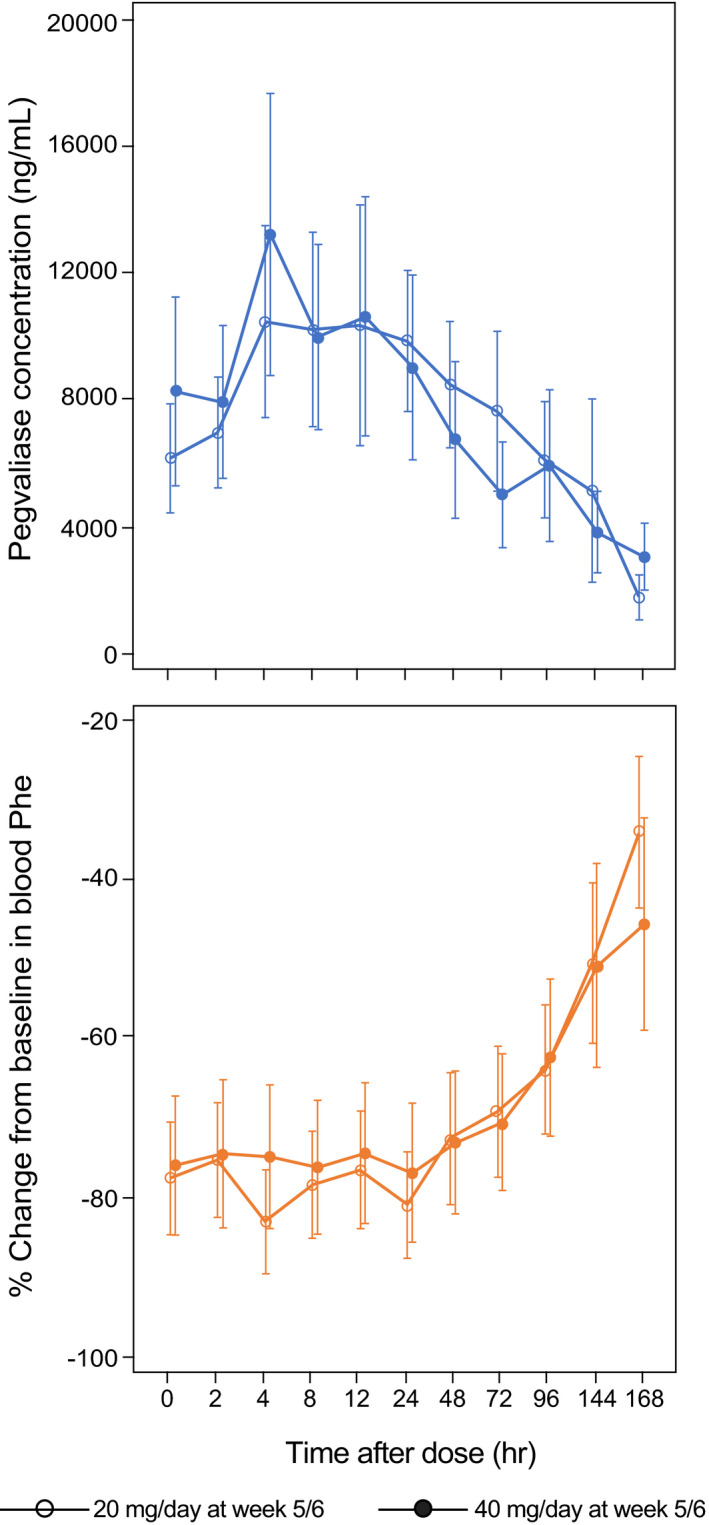
Intensive PK/PD profile of plasma pegvaliase and Phe concentrations in the open‐label phase III PRISM‐2 study in adults with PKU who received pegvaliase 20 or 40 mg/day and achieved at least a 20% decrease from baseline in blood Phe levels. Intensive PK/PD sampling was performed during week 1 and weeks 5–6 in part 3 of PRISM‐2 (N = 57). Data are mean ± standard error. PD, pharmacodynamic; Phe, phenylalanine; PK, pharmacokinetic; PKU, phenylketonuria
PK and PD over the 24‐h dosing interval
Steady‐state pegvaliase PK was characterized using intensive PK samples collected in part 3 of PRISM‐2, when patients were receiving randomized maintenance doses of pegvaliase 20 or 40 mg/day. A relatively flat steady‐state profile, with a low peak‐to‐trough ratio, was observed in plasma pegvaliase and blood Phe concentrations during the 24‐h dosing interval (Figure 3, Table 1). The mean terminal half‐life of pegvaliase was 47.3 ± 41.6 h and 60.2 ± 44.6 h in the 20 and 40 mg/day dose groups, respectively. The mean pegvaliase C trough were 11.2 ± 9.0 mg/L and 10.4 ± 12.7 mg/L in the 20 and 40 mg/day dose groups, respectively. The long half‐life of pegvaliase resulted in a low intrasubject fluctuation of plasma pegvaliase and blood Phe levels during the 24‐h dosing interval, which showed that trough blood Phe levels are a good indication of the steady‐state level for long‐term treatment. Similar to other studies, an inverse correlation between plasma pegvaliase concentrations and blood Phe level reduction was also observed here. In general, for patients with pegvaliase C trough greater than or equal to 10,000 ng/ml, their blood Phe levels remained stable below 30 µmol/L during the 24‐h dosing interval. Fifteen of 47 patients had at least one nonquantifiable blood Phe level (<2 µmol/L) during the 24‐h dosing interval as a result of high plasma pegvaliase concentration. Among these 15 patients, blood Phe levels of 5 patients remained nonquantifiable during the 24 h.
TABLE 1.
Steady‐state pegvaliase PK parameters in PRISM‐2, weeks 5–6 of maintenance phase (part 3)
| Parameter | Statistic | Stayed on active treatment | Switched from placebo | ||
|---|---|---|---|---|---|
|
20 mg/day n = 20 |
40 mg/day n = 18 |
20 mg/day n = 11 |
40 mg/day n = 8 |
||
| AUC0–24 (ng/hr/ml) | n | 17 | 12 | 7 | 7 |
| Mean (SD) | 262,181.5 (280,378.2) | 246,782.5 (338,587.5) | 116,564.8 (111,571.1) | 454,920.8 (649,658.7) | |
| Range | 5470–1,126,030 | 4507–1,169,209 | 10,583–319,763 | 5482–1,425,093 | |
| Cmax (ng/ml) | n | 17 | 15 | 8 | 8 |
| Mean (SD) | 14,042.6 (16,254.7) | 16,687.3 (19,457.1) | 5334.5 (5209.9) | 23,053.8 (35,974.3) | |
| Range | 255–68,500 | 239–63,800 | 536–15,500 | 91–91,500 | |
| Ctrough (ng/ml) | n | 15 | 14 | 8 | 8 |
| Mean (SD) | 11,177.3 (8974.7) | 10,446.0 (12,723.9) | 4995.9 (5216.1) | 17,408.5 (29,014.3) | |
| Range | 210–29,600 | 176–43,100 | 347–15,500 | 0–66,100 | |
| Tmax (hour) | n | 17 | 15 | 8 | 8 |
| Mean (SD) | 9.8 (8.1) | 7.5 (4.6) | 16.6 (7.4) | 8.4 (7.8) | |
| Range | 0–24 | 0–12 | 8–24 | 0–24 | |
| t1/2 (hour) | n | 13 | 8 | 5 | 3 |
| Mean (SD) | 47.3 (41.6) | 60.2 (44.6) | 49.6 (37.9) | 26.5 (11.9) | |
| Range | 14–132 | 14–127 | 15–115 | 14–37 | |
Abbreviations: AUC0–24, area under the cure from 0 to 24 hours; Cmax, maximum concentration; Ctrough, trough blood pegvaliase concentrations; PK, pharmacokinetic; SD, standard deviation; t1/2, time to 50% reduction in Cmax; Tmax, time to reach Cmax.
PK and immunogenicity
All the patients in the studies reported here tested positive for anti‐PAL antibody responses, and most developed transient anti‐PEG antibody responses. 19 , 21 , 22 , 23 , 24 , 25 , 26 In the 24‐week study 165–205, the 11 patients who achieved a maintenance dose (5–75 mg/day, required to maintain blood Phe ≤600 µmol/L) generally had lower ADA positivity rates and lower mean ADA titers than those who did not achieve a maintenance dose during the study. 24 The majority of patients who did not achieve significant blood Phe level reduction in the 24‐week study were able to attain blood Phe lowering in the long‐term extension study, which allowed titration to higher doses to overcome the immune‐mediated clearance. 24
The immune response to pegvaliase varied substantially among patients during PRISM‐1 (Figure 4) and was inversely related to drug concentrations, resulting in highly variable pegvaliase C trough among patients, irrespective of drug dose at 20 or 40 mg/day. Although high individual variability in immune response resulted in substantial overlap between ADA quartiles, in general, patients in the lowest antibody titer quartile had the highest mean pegvaliase C trough. All patients in PRISM‐1 developed anti‐pegvaliase and anti‐PAL antibody responses that peaked during early treatment (≤6 months after treatment initiation), then sustained through week 36 of PRISM‐1 and throughout PRISM‐2 (mean at week 73: total antibody titers, 18,924; anti‐PAL IgG titers, 804,555; anti‐PAL IgM titers, 2714). Anti‐PEG responses also peaked during early treatment in PRISM‐1, returning to near baseline levels by study end, where they remained throughout the duration of PRISM‐2 (week 73: anti‐PEG IgG, 11; anti‐PEG IgM, 62). Neutralizing antibodies were detected in 77% of patients at week 36, with titers peaking at 540 during week 16 and remaining steady (mean: 509) through week 36 of PRISM‐1 and throughout PRISM‐2 (week 73 of part 4: 442). In PRISM‐2, an inverse correlation between ADA responses and pegvaliase Ctrough was also observed, which was consistent with the results from earlier studies. This further confirmed that pegvaliase clearance is primarily driven by an immune response after repeat dosing.
FIGURE 4.
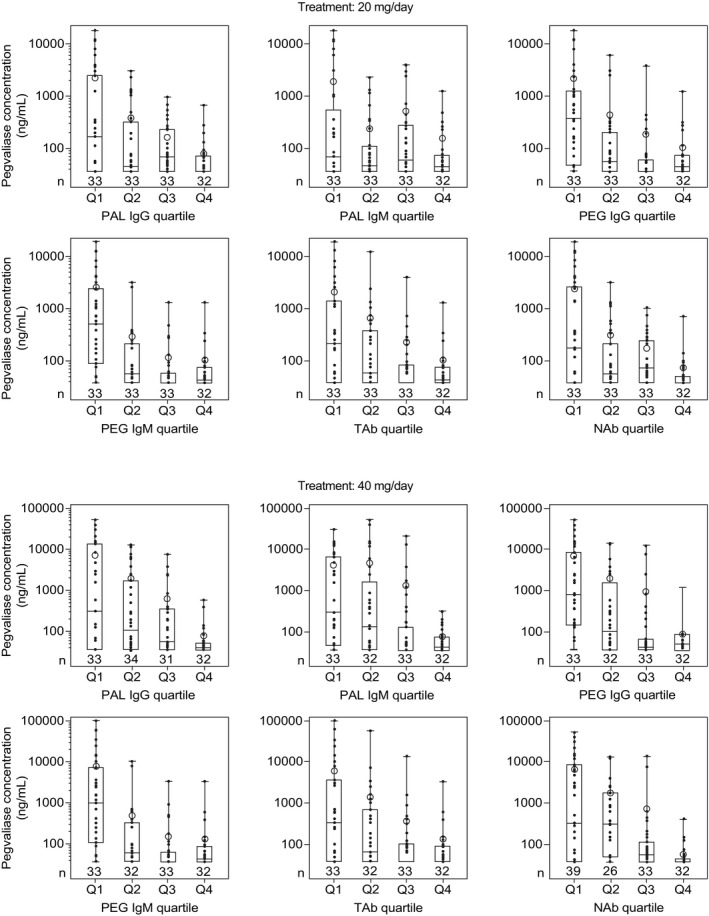
Mean pegvaliase C trough according to antibody titer quartiles at 20 mg/day in PRISM‐1 (N = 131). Samples were collected on days 1 and 22 during the induction period and every 4 weeks during titration and maintenance. C trough, trough plasma concentration; IgG, immunoglobulin G; NAbs, neutralizing antibodies; PAL, phenylalanine ammonia lyase; PEG, polyethylene glycol; TAbs, total antibodies
Effects of dietary Phe intake on the PK/PD relationship
The effects of dietary Phe intake on the PK/PD relationship between plasma pegvaliase and Phe levels were assessed by matching pegvaliase C trough to time‐matched blood Phe levels according to quartiles of dietary Phe intake (Figure 5, Table S2). Dietary Phe intake was measured on the same day or the day before PK/PD sampling. In PRISM‐1 and PRISM‐2, dietary Phe intake was positively correlated with blood Phe levels for patients with pegvaliase C trough less than 10,000 ng/ml, but not for those with pegvaliase C trough greater than or equal to 10,000 ng/ml. Among patients with pegvaliase C trough greater than or equal to 10,000 ng/ml, 96.7% of blood Phe levels remained below 30 µmol/L regardless of dietary Phe intake. As dietary Phe intake increased from the lowest quartile (Q1) to the highest (Q4), a significant right shift of the PK/PD relationship was observed (p < 0.05 for difference in estimated mean IC50; values for the quartile 1 [Q1] and quartile 4 [Q4] groups, t‐test), which suggested that an increase in dietary Phe intake could increase the blood Phe level in individuals treated with pegvaliase when pegvaliase C trough are not very high (i.e., <10,000 ng/ml).
FIGURE 5.
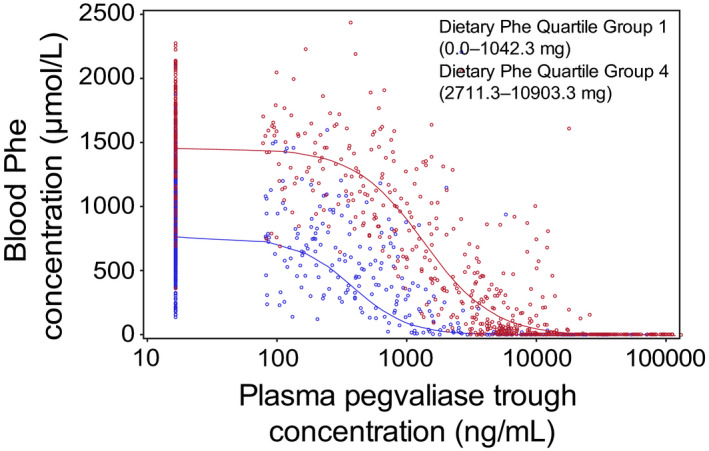
Pegvaliase C trough versus Phe level, according to dietary Phe intake in PRISM‐1 and PRISM‐2 (N = 260). The reportable range of blood Phe concentration was 5–3750 µmol/L. Phe intake (including intact food and medical food) was measured on the same day or the day before PK and PD sampling was performed. Blood Phe levels were affected by dietary Phe intake among patients with pegvaliase C trough less than 10,000 ng/ml; in contrast, when pegvaliase C trough was greater than or equal to 10,000 ng/mL, dietary Phe intake had no impact on blood Phe concentration, even among patients with the highest dietary Phe intake. Quartiles of dietary Phe intake: quartile 1 (Q1), 0–1042.3 mg; quartile 4 (Q4), 2711.3–10,903.3 mg. C trough, trough plasma concentration; Phe, phenylalanine; PD, pharmacodynamic; PK, pharmacokinetic
PK and safety
High plasma pegvaliase concentrations were not associated with incidence of HAEs, SAEs, anaphylaxis events, or injection site reactions in the PRISM studies. Patients who experienced fewer HAEs during the induction and titration phases of PRISM‐1 and those without SAEs or anaphylaxis typically had higher pegvaliase C trough and lower ADA titers during the induction/titration phases (Figure 6). Patients with higher pegvaliase C trough typically had lower ADA titers and a more favorable tolerability profile. Overall, plasma pegvaliase levels were nonquantifiable during the first 8 weeks of PRISM‐1 and were generally low during induction/titration, when administered doses of pegvaliase were low and both circulating immune complex concentrations and HAEs were peaking. Over time, the frequency of HAEs decreased as circulating immune complex concentrations decreased, and pegvaliase concentrations increased as higher pegvaliase doses were administered, suggesting that development of HAEs was temporally associated with immune response rather than pegvaliase plasma levels.
FIGURE 6.
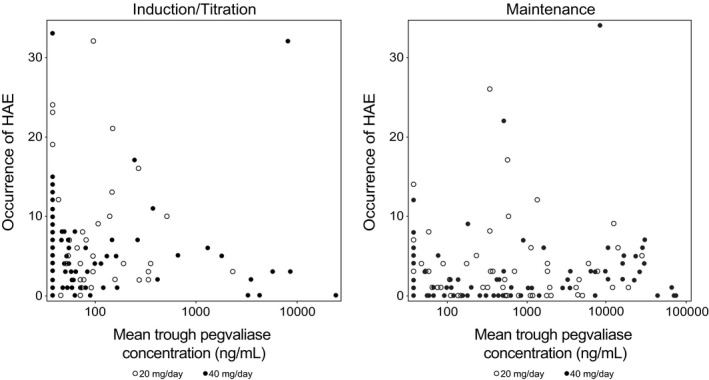
Correlation between pegvaliase C trough and occurrence of HAEs in the I/T/M periods of the PRISM‐1 study by randomized dose (PK population). Occurrences of HAEs are the total numbers of events during each study period. Mean pegvaliase C trough is the mean pegvaliase C trough in each study period. C trough, trough plasma concentration; HAE, hypersensitivity adverse event; I/T/M, induction/titration/maintenance; PK, pharmacokinetic
DISCUSSION
The results of these studies demonstrate that immunogenicity substantially impacts pegvaliase PKs. Because pegvaliase is a foreign, bacterially derived protein, drug administration triggers the development of Abs against the drug, leading to the formation of ADA immune complexes, complement activation, and immune‐mediated clearance of the drug. 12 , 13 , 14 , 15 These results demonstrated that both the trajectory and the magnitude of the ADA response influenced the drug effect on reduction of blood Phe levels. Subjects with higher ADA titers required higher doses of pegvaliase to overcome clearance and achieve efficacious plasma concentrations, leading to optimal blood Phe level reduction. 21 Because of the individualized immune response to the drug, titration of pegvaliase to the lowest effective dosage based on patient tolerability and Phe‐lowering efficacy is necessary.
An I/T/M dosing regimen was developed to minimize the safety impact of ADA responses and maximize the blood Phe‐lowering clinical benefit of pegvaliase. During the early treatment phase, anti‐PEG IgG/IgM and anti‐PAL IgM antibody responses peaked, forming circulating immune complexes that activated the complement pathway and induced immune‐mediated clearance of the drug. The anti‐PEG antibodies of the early response can readily bind to the unobscured PEG epitopes on the surface of the drug product and form immune complexes that efficiently activate the classical complement pathway. In addition, due to its pentameric structure, IgM can efficiently form immune complexes and thereby readily activate the classical complement pathway. 14 , 27 , 28 The circulating immune complexes of pegvaliase retain enzymatic activity prior to clearance, suggesting that antibody binding does not completely block the active site on pegvaliase. Therefore, total pegvaliase concentrations consisting of free and ADA‐bound pegvaliase were measured in the clinical studies.
As the immune response matured during late treatment (>6 months after treatment initiation), higher pegvaliase doses and slower drug clearance contributed to higher plasma pegvaliase concentrations. The immune response during late treatment consisted primarily of anti‐PAL IgG. Immune complex formation, and therefore immune‐mediated drug clearance, was reduced during this time, likely because anti‐PAL Abs cannot bind efficiently to the drug product due to epitope masking of the PAL protein by the extensive PEGylation.
Neutralizing antibodies were also observed in PRISM‐1 and PRISM‐2. Although a majority of patients developed sustained neutralizing antibody responses in PRISM‐1 and PRISM‐2, most of these patients were able to reach sustained efficacy. As the neutralizing antibody responses apparently were not able to effectively neutralize the enzymatic activity of the drug, the clinical importance of neutralizing antibodies was limited. In long‐term treatment with pegvaliase in clinical trials, blood Phe level reductions were sustained because the high pegvaliase plasma concentration achieved by individualized pegvaliase dose levels overcame the impact of the low‐titer neutralizing antibody response. An extension of both study 165–205 and PRISM‐2 supported this finding, in which blood Phe level reductions were sustained during stable neutralizing antibody responses that developed during treatment. 21 , 24
Because plasma pegvaliase exposure was predominantly affected by ADAs and body weight did not show a significant effect, pegvaliase administration was changed from body weight‐adjusted dosing in earlier clinical studies to fixed dosing in later clinical studies (Figure S1). In PRISM‐1, patients were randomized 1:1 to titrate to a maintenance dose of 20 or 40 mg/day. Patients unable to achieve optimal efficacy at the 20 or 40 mg/day doses were transitioned to part 4 of PRISM‐2, in which dose adjustments were permitted, up to 60 mg/day in some cases, in order to reach a Phe‐lowering response.
The PK/PD model characterized the effects of dietary Phe on the relationship between pegvaliase PK and PD observed in PRISM‐1 and PRISM‐2—namely, that increased protein intake correlated with increased blood Phe levels for patients with pegvaliase C trough less than 10,000 ng/ml. This information is helpful for physicians to conduct dose adjustment based on Phe levels and dietary Phe intake. Although patients were instructed to maintain a stable protein intake, study protocols allowed increased dietary protein intake if blood Phe levels dropped to less than or equal to 30 µmol/L. In clinical practice, these data support increasing dietary Phe intake for patients who develop low blood Phe levels (<30 µmol/L) if the patient is not already consuming the recommended daily level of protein. If the patient’s blood Phe level does not increase to greater than 30 µmol/L after increasing dietary Phe intake, the pegvaliase dose should then be reduced.
Dose recommendations for pegvaliase reflect the findings from the studies reported here, in that patients are individually titrated to reach the lowest effective dosage based on tolerability and Phe‐lowering efficacy. 10 A slow upward I/T/M dosing regimen of pegvaliase can lead to sustained efficacy with improved tolerability over time in patients with PKU.
CONCLUSION
The PK, PD, and immunogenicity results reported here provide a rationale for the pegvaliase I/T/M dosing regimen for the treatment of adults with PKU. The results of these studies demonstrate that immunogenicity plays a prominent role in pegvaliase PK. However, the I/T/M dosing regimen has been shown to diminish the effects of immunogenicity in this population, and patients achieve substantial blood Phe level reduction and increase their dietary protein intake with improved tolerability over time. This dosing strategy may provide a method for administration of other biologic drugs of nonhuman origin.
DATA SHARING STATEMENT
The de‐identified individual participant data that underlie the results reported in this article (including text, tables, figures, and supplementary files) will be made available together with the research protocol and data dictionaries, for noncommercial, academic purposes.
Additional supporting documents may be available upon request.
Investigators will be able to request access to these data and supporting documents via a data sharing portal beginning 6 months and ending 2 years after publication. Data associated with any ongoing development program will be made available within six (6) months after approval of the relevant product.
Requests must include a research proposal clarifying how the data will be used, including proposed analysis methodology.
Research proposals will be evaluated relative to publicly available criteria available at www. BioMarin.com/patients/publication‐data‐request/ to determine if access will be given, contingent upon execution of a data access agreement with BioMarin Pharmaceutical Inc.
CONFLICTS OF INTEREST
Y.Q., G.P., J.H., S.G., J.O., K.L., M.M., M.L., Z.G., S.J.Z., H.H.W., and B.S. are employees and shareholders of BioMarin. C.O.H. and N.L. have received grants and personal fees from BioMarin. R.Z. has received speaker fees, consulting fees, and travel support from BioMarin. B.K.B. has received speaker and consulting fees from BioMarin.
AUTHOR CONTRIBUTIONS
Y.Q., G.P., J.H., S.G., J.O., K.L., M.M., M.L., Z.G., S.J.Z., H.H.W., and B.S. wrote the manuscript. Y.Q., S.G., J.O., M.M., Z.G., H.H.W., B.S., and J.H. designed the research. C.O.H., R.Z., N.L., and B.K.B. performed the research. Y.Q., G.P., J.H., S.G., J.O., K.L., M.M., M.L., Z.G., S.J.Z., H.H.W., and B.S. analyzed the data. K.L. and S.J.Z. contributed new analytical tools.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the patients and physicians who participated in these studies. Autumn Kelly, MA (Ashfield Healthcare Communications, Middletown, CT, USA) drafted and revised the manuscript based on input from authors, and Erin Spohr and Mary Kacillas (Ashfield Healthcare Communications) copyedited and styled the manuscript per journal requirements.
Funding information
This work was supported by BioMarin Pharmaceutical Inc., Novato, California.
REFERENCES
- 1. Burton BK, Grange DK, Milanowski A, et al. The response of patients with phenylketonuria and elevated serum phenylalanine to treatment with oral sapropterin dihydrochloride (6R‐tetrahydrobiopterin): a phase II, multicentre, open‐label, screening study. J Inherit Metab Dis. 2007;30:700‐707. [DOI] [PubMed] [Google Scholar]
- 2. Bilder DA, Noel JK, Baker ER, et al. Systematic review and meta‐analysis of neuropsychiatric symptoms and executive functioning in adults with phenylketonuria. Dev Neuropsychol. 2016;41:245‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. ten Hoedt AE, de Sonneville LM , Francois B, et al. High phenylalanine levels directly affect mood and sustained attention in adults with phenylketonuria: a randomised, double‐blind, placebo‐controlled, crossover trial. J Inherit Metab Dis. 2011;34:165‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jurecki ER, Cederbaum S, Kopesky J, et al. Adherence to clinic recommendations among patients with phenylketonuria in the United States. Mol Genet Metab. 2017;120:190‐197. [DOI] [PubMed] [Google Scholar]
- 5. Walter JH, White FJ, Hall SK, et al. How practical are recommendations for dietary control in phenylketonuria? Lancet. 2002;360:55‐57. [DOI] [PubMed] [Google Scholar]
- 6. Kuvan (sapropterin dihydrochloride) [prescribing information]. Novato, CA: BioMarin Pharmaceutical Inc., 2014. [Google Scholar]
- 7. Trefz F, Burton BK, Longo N, et al. Efficacy of sapropterin dihydrochloride in increasing phenylalanine tolerance in children with phenylketonuria: a phase III, randomized, double‐blind, placebo‐controlled study. J Pediatr. 2009;154:700‐707. [DOI] [PubMed] [Google Scholar]
- 8. Bell SM, Wendt DJ, Zhang Y, et al. Formulation and PEGylation optimization of the therapeutic PEGylated phenylalanine ammonia lyase for the treatment of phenylketonuria. PLoS One. 2017;12:e0173269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levy HL, Sarkissian CN, Scriver CR. Phenylalanine ammonia lyase (PAL): from discovery to enzyme substitution therapy for phenylketonuria. Mol Genet Metab. 2018;124:223‐229. [DOI] [PubMed] [Google Scholar]
- 10. Palynziq (pegvaliase‐pqpz) [package insert]. Novato, CA: BioMarin Pharmaceutical Inc., 2020. [Google Scholar]
- 11. Palynziq (pegvaliase‐pqpz) [SmPC or Summary of Product Characteristics]. European Medicines Agency. https://www.ema.europa.eu/en/documents/product‐information/palynziq‐epar‐product‐information_en.pdf. Accessed September 2020. [Google Scholar]
- 12. Chirmule N, Jawa V, Meibohm B. Immunogenicity to therapeutic proteins: impact on PK/PD and efficacy. AAPS J. 2012;14:296‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koren E, Smith HW, Shores E, et al. Recommendations on risk‐based strategies for detection and characterization of antibodies against biotechnology products. J Immunol Methods. 2008;333:1‐9. [DOI] [PubMed] [Google Scholar]
- 14. Krishna M, Nadler SG. Immunogenicity to biotherapeutics ‐ the role of anti‐drug immune complexes. Front Immunol. 2016;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang YM, Jawa V, Ma M. Immunogenicity and PK/PD evaluation in biotherapeutic drug development: scientific considerations for bioanalytical methods and data analysis. Bioanalysis. 2014;6:79‐87. [DOI] [PubMed] [Google Scholar]
- 16. Hu CF, Sakamoto KM. PEG‐asparaginase. Expert Opin Pharmacother. 2007;8:1977‐1984. [DOI] [PubMed] [Google Scholar]
- 17. Hoffman RM, Yang Z, Tan Y, Han Q, Li S, Yagi S. Safety and toxicity of recombinant methioninase and polyethylene glycol (PEG) recombinant methioninase in primates. Methods Mol Biol. 2019;1866:211‐229. [DOI] [PubMed] [Google Scholar]
- 18. Walsh S, Shah A, Mond J. Improved pharmacokinetics and reduced antibody reactivity of lysostaphin conjugated to polyethylene glycol. Antimicrob Agents Chemother. 2003;74:554‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Longo N, Harding CO, Burton BK, et al. Single‐dose, subcutaneous recombinant phenylalanine ammonia lyase conjugated with polyethylene glycol in adult patients with phenylketonuria: an open‐label, multicentre, phase 1 dose‐escalation trial. Lancet. 2014;384:37‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang P, Sun F, Liu S, Jiang S. Anti‐PEG antibodies in the clinic: current issues and beyond PEGylation. J Control Release. 2016;244:184‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gupta S, Lau K, Harding CO, et al. Association of immune response with efficacy and safety outcomes in adults with phenylketonuria administered pegvaliase in phase 3 clinical trials. EBioMedicine. 2018;37:366‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thomas J, Levy H, Amato S, et al. Pegvaliase for the treatment of phenylketonuria: results of a long‐term phase 3 clinical trial program (PRISM). Mol Genet Metab. 2018;124:27‐38. [DOI] [PubMed] [Google Scholar]
- 23. Thomas JA, Longo N, Zori R, et al. Evaluation of multiple dosing regimens in phase 2 studies of rAvPAL‐PEG (BMN 165, pegvaliase) for control of blood phenylalanine levels in adults with phenylketonuria [poster]. Presented at SIMD Annual Meeting; March 28–31, 2015; Salt Lake City, UT.
- 24. Zori R, Thomas JA, Shur N, et al. Induction, titration, and maintenance dosing regimen in a phase 2 study of pegvaliase for control of blood phenylalanine in adults with phenylketonuria. Mol Genet Metab. 2018;125:217‐227. [DOI] [PubMed] [Google Scholar]
- 25. Harding CO, Amato RS, Stuy M, et al. Pegvaliase for the treatment of phenylketonuria: a pivotal, double‐blind randomized discontinuation phase 3 clinical trial. Mol Genet Metab. 2018;124:20‐26. [DOI] [PubMed] [Google Scholar]
- 26. Longo N, Zori R, Wasserstein MP, et al. Long‐term safety and efficacy of pegvaliase for the treatment of phenylketonuria in adults: combined phase 2 outcomes through PAL‐003 extension study. Orphanet J Rare Dis. 2018;13:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20:34‐50. [DOI] [PubMed] [Google Scholar]
- 28. Eisen HN. Affinity enhancement of antibodies: how low‐affinity antibodies produced early in immune responses are followed by high‐affinity antibodies later and in memory B‐cell responses. Cancer Immunol. Res. 2014;2:381‐392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


