FIGURE 3.
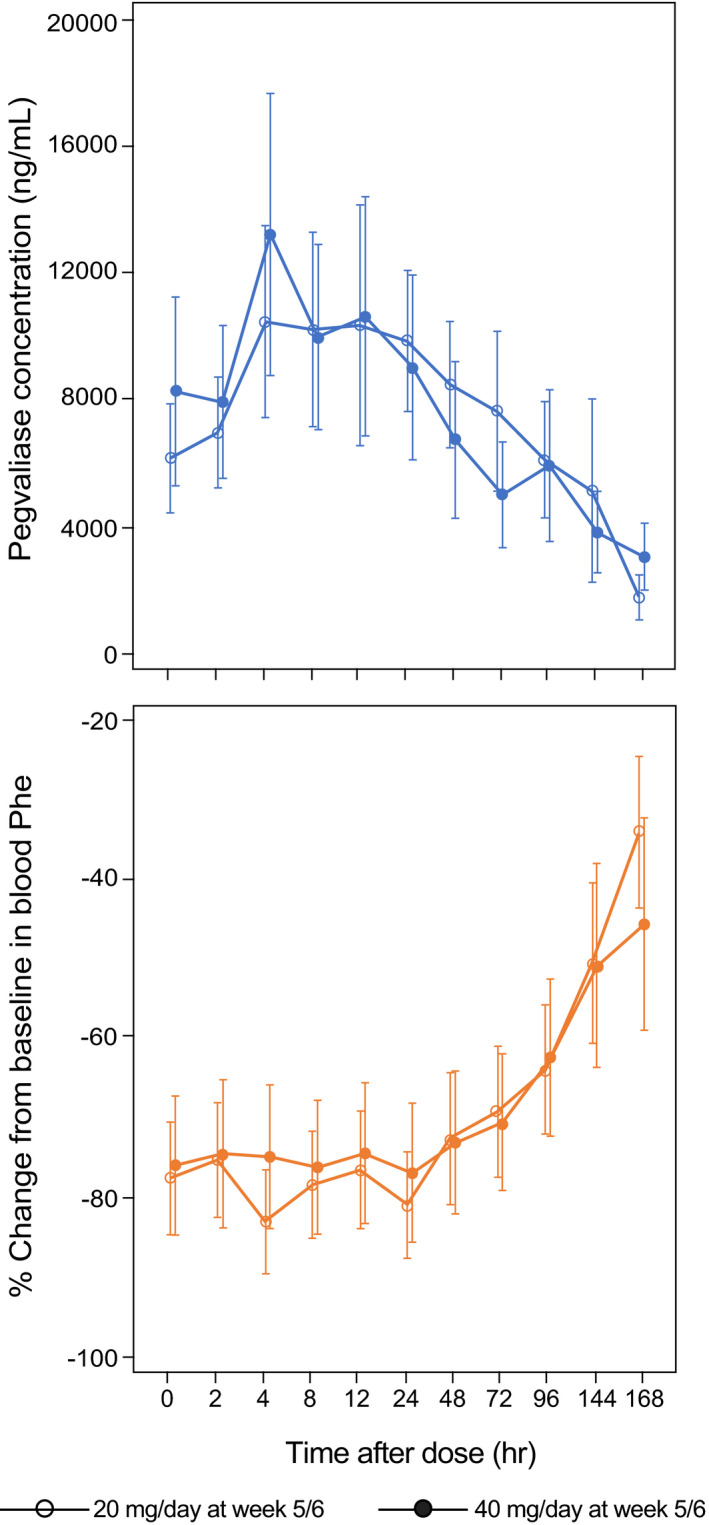
Intensive PK/PD profile of plasma pegvaliase and Phe concentrations in the open‐label phase III PRISM‐2 study in adults with PKU who received pegvaliase 20 or 40 mg/day and achieved at least a 20% decrease from baseline in blood Phe levels. Intensive PK/PD sampling was performed during week 1 and weeks 5–6 in part 3 of PRISM‐2 (N = 57). Data are mean ± standard error. PD, pharmacodynamic; Phe, phenylalanine; PK, pharmacokinetic; PKU, phenylketonuria
