Abstract
Aims/Introduction
To examine the prevalence of falls and fractures, and the association with symptoms of diabetic polyneuropathy (DPN) in patients with recently diagnosed type 2 diabetes.
Materials and Methods
A detailed questionnaire on neuropathy symptoms and falls was sent to 6,726 patients enrolled in the Danish Center for Strategic Research in Type 2 Diabetes cohort (median age 65 years, diabetes duration 4.6 years). Complete data on fractures and patient characteristics were ascertained from population‐based health registries. We defined possible DPN as a score ≥4 on the Michigan Neuropathy Screening Instruments questionnaire. Using Poisson regression analyses, we estimated the adjusted prevalence ratio (aPR) of falls and fractures, comparing patients with and without DPN.
Results
In total, 5,359 patients (80%) answered the questions on the Michigan Neuropathy Screening Instruments questionnaire and falls. Within the year preceding the questionnaire response, 17% (n = 933) reported at least one fall and 1.4% (n = 76) suffered from a fracture. The prevalence ratio of falls was substantially increased in patients with possible DPN compared with those without (aPR 2.33, 95% confidence interval [CI] 2.06–2.63). The prevalence ratio increased with the number of falls from aPR 1.51 (95% CI 1.22–1.89) for one fall to aPR 5.89 (95% CI 3.84–9.05) for four or more falls within the preceding year. Possible DPN was associated with a slightly although non‐significantly increased risk of fractures (aPR 1.32, 95% CI 0.75–2.33).
Conclusions
Patients with recently diagnosed type 2 diabetes and symptoms of DPN had a highly increased risk of falling. These results emphasize the need for preventive interventions to reduce fall risk among patients with type 2 diabetes and possible DPN.
Keywords: Diabetic polyneuropathy, Falls, Fractures
Patients with recently diagnosed type 2 diabetes and symptoms of diabetic polyneuropathy had a highly increased risk of falling, whereas possible diabetic polyneuropathy was only slightly and non‐significantly associated with fractures. Identifying patients with possible diabetic polyneuropathy might help in the identification of patients at risk of falls and should be considered in future longitudinal studies on fall prevention.

Introduction
The risk of falls is increased in patients with diabetes 1 , 2 , and falling is a major cause of morbidity and mortality in the elderly 3 , 4 . Patients with type 2 diabetes suffer from more recurrent falls 5 , more severe fall‐related injuries 6 , a higher risk of bone fractures 7 and increased risk of hospitalized fall injury 8 compared with healthy individuals. Delayed fracture healing in diabetes 9 leads to longer hospital stays and more frequent hospital readmissions 10 , which poses a great economic burden on the healthcare system 5 . Therefore, knowledge on the causes of falls and fractures, and the identification of persons with high fall risk early in the course of type 2 diabetes is of great importance.
Diabetic polyneuropathy (DPN) is a common complication in type 2 diabetes 11 that leads to decreased peripheral sensation 12 , unstable gait 13 , impaired balance 14 and motor dysfunction 15 . A few studies have suggested a positive association of DPN in type 2 diabetes patients with falls 16 , 17 , 18 , 19 , fractures 20 , 21 , 22 , 23 , or both 24 , 25 , but convincing evidence is lacking, as the studies have been limited by either size (≤48 patients) 17 , 23 , by not applying validated tools for DPN assessment 18 , 20 , 22 , 24 , 25 , by including mixed diabetes populations (both type 1 and type 2 diabetes) 20 , by lacking a comparisons group without DPN 17 or by including older populations (age 70–79 years) 16 . We, therefore, examined the prevalence of falls and fractures in a large cohort of patients with recently diagnosed type 2 diabetes in Denmark including both patients with and without DPN. We hypothesized that symptoms of DPN are associated with an increased risk of falls and fractures.
Materials and Methods
The present nationwide cross‐sectional questionnaire study was carried out on patients enrolled in the Danish Center for Strategic Research in Type 2 Diabetes (DD2) project cohort (https://dd2.dk/). The DD2 cohort includes enrolls recently diagnosed type 2 patients from general practitioners and endocrinology clinics in Denmark. Enrollment began in November 2010 and is still ongoing 26 . Details on the implementation, enrolment process, data linkage and patient characteristics of this cohort have been described in detail elsewhere 27 .
Study population
On 7 June 2016, a questionnaire consisting of 41 items was sent to all patients enrolled in the DD2 cohort (n = 6,726). The questionnaire was sent to all non‐responders a second time on 12 September 2016, and a third time on 10 October 2016. The questionnaire has been described in detail elsewhere 28 . The present study population consisted of the patients who answered both the questions on falls and the Michigan Neuropathy Screening Instrument Questionnaire (MNSIq). Figure S1 shows a flowchart of the study population.
DPN
The MNSIq is a self‐administered validated screening tool available for DPN assessment. The questionnaire consists of 15 ‘yes’ or ‘no’ questions with a maximal score of 13. We used the MNSIq for the evaluation of symptoms of DPN with a validated cut‐off score ≥4 to define DPN at the level of ‘possible’ DPN according to the Toronto Classification of DPN 11 .
Falls
In the questionnaire, patients were asked to report whether they had fallen within the preceding year, and if so, how many fall episodes they had experienced: none, one, two to four or more than four falls. This method of fall evaluation has been used in previous studies evaluating fall frequency 19 , and is the best time frame for obtaining self‐reported falls ruling out any seasonal influence 29 . Patients were asked whether the fall episode(s) had led them to seek medical attention, and to specify whether they contacted their general practitioner and/or a hospital.
Fractures
As the questionnaire did not contain questions regarding fractures, we extracted data on fractures from the Danish National Patient Register (DNPR) 30 . All Danish residents are assigned a unique personal identification number, which allows for accurate individual‐level linkage of data across registries 27 , including the DNPR. The DNPR 30 includes recorded data regarding all non‐psychiatric hospital admissions since 1977, and on outpatient clinic and emergency room visits since 1995. In Denmark, all persons suspected of having a fracture are referred to the hospital for further diagnostic work‐up. Fractures are coded according to the International Classification of Diseases, version 10 from 1994 onwards; thus, the DNPR holds complete fracture history. We extracted data on fractures, including the number and types of fractures for all individuals included in the study (n = 5,359). Data on fractures were linked and time‐matched to the questionnaire data on the occurrence of falls within the preceding year. Figure 1 shows the dates on which the questionnaires were sent out, and the corresponding time periods used for the assessment of falls and fractures in the primary analyses. The precise date on which the questionnaires were filled out was not obtained. Thus, in a sensitivity analysis, we expanded the period from 7 June 2015 to 24 January 2017, which was the date the last questionnaire was returned, to also include possible fractures in the period from 10 October 2016 to 24 January 2017, as shown in Figure 1. Fractures were identified based on pre‐specified International Classification of Diseases, version 10 codes (Table S1). If the same type of fracture occurred within 3 months from the prior fracture, we assumed that this represented the same fracture and consequently was counted once only (see Table S1 for codes and definitions).
Figure 1.
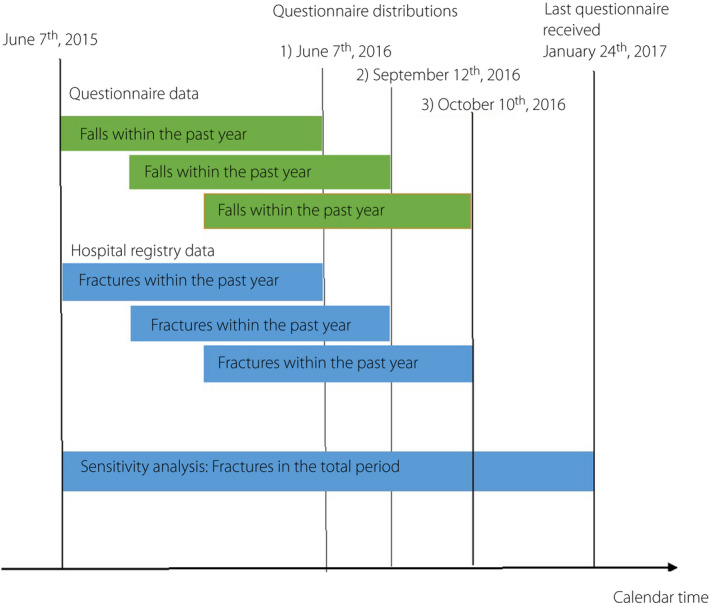
Dates for the questionnaire distributions and the corresponding time period used for the assessment of falls and fractures. Orange boxes represent time periods over which falls were assessed in the questionnaire. Blue boxes show the corresponding periods used for data extraction from the registry and represent time period over which fractures were obtained. The sensitivity analysis included the entire time period until 24 January 2017.
Other characteristics
We obtained information from the questionnaire regarding other patient characteristics that might be associated with falls and fractures, including age, sex, body mass index (BMI), alcohol consumption and smoking. Additional data, such as the presence of eye disease and a range of comorbidities included in the Charlson Comorbidity Index (CCI), was extracted from the DNPR. Data on medication use were attained from the DNPR. Diabetes duration was determined by either the first diabetes‐related hospital contact, the first prescription redemption of a glucose‐lowering drug or enrollment in DD2. Codes used for the additional data extraction are presented in Table S2. Based on the overall CCI score excluding diabetes, the comorbidity burden was divided into three categories: no comorbidity (CCI score 0), moderate comorbidity (CCI score 1–2) and high comorbidity (CCI score ≥3) 31 .
Ethical considerations
All patients in the DD2 project gave informed written consent, and the project was approved by the National Danish Committee on Health Research Ethics (S‐20100082) and registered with the Data Protection Agency (2008‐58‐0035).
Statistical analysis
The main outcomes were self‐reported falls and registry‐based fractures within the year preceding DPN assessment. We calculated the prevalence of falls and fractures, and used Poisson regression to calculate prevalence ratios (PRs) with 95% confidence intervals (95% CI) of falls and fractures for patients with DPN compared to patients without DPN, adjusting for potential confounders mentioned above. We stratified the analyses according to biological sex. Moreover, we conducted a more extensively adjusted analysis adding use of antihypertensive medication and insulin to the regression model. The adjusted PRs (aPR) of falls comparing patients with DPN with those without were evaluated by the number of falls. We further compared the proportions of patients with falls who sought medical attention, and we assessed the subtype and number of fractures.
Statistical analyses were carried out using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Among the 6,726 DD2 patients, who received a questionnaire, 5,359 (80%) answered the questions on falls and the MNSIq (Figure S1). Of these, 17% (n = 933) reported at least one fall within the preceding year. Out of all patients reporting at least one fall, 46% had suffered from one fall, 36% had two to four falls and 10% had more than four falls, whereas 8% did not specify the number of falls. In total, 1.4% (n = 76) had suffered at least one fracture within the preceding year.
DPN and falls
In patients with DPN, after adjustment for confounders, the prevalence of falls was substantially higher as compared with those without DPN (aPR 2.33; 95% confidence interval [CI] 2.06–2.63; Table 1), which was found for both women and men (Table S3). An additional adjustment for the use of antihypertensive medication and insulin did not change the estimate (aPR 2.31, 95% CI 2.04–2.61; Table S4). The prevalence ratio increased with the number of falls from aPR 1.51 (95% CI 1.22–1.89) for having precisely one fall in patients with DPN compared with those without DPN, aPR 2.86 (95% CI 2.32–3.52) for having two to four falls and aPR 5.89 (95% CI 3.84–9.05) for having four or more falls within the preceding year (Table 2).
Table 1.
Prevalence of falls and fractures in patients with type 2 diabetes, and the adjusted prevalence ratios of falls and fractures associated with possible diabetic polyneuropathy and clinical characteristics
| Falls | Fractures | |||||
|---|---|---|---|---|---|---|
|
No falls n (%) ‡ |
≥1 Fall n (%) ‡ |
≥1 Fall aPR (95% CI) § |
No fractures n (%) ‡ |
≥1 Fracture n (%) ‡ |
≥1 Fracture aPR (95% CI) § |
|
| Total | 4,426 (82.5) | 933 (17.4) | – | 5,283 (98.6) | 76 (1.4) | – |
| Possible DPN † | ||||||
| No | 3,790 (86.2) | 607 (13.8) | Ref. | 4,337 (98.6) | 60 (1.4) | Ref. |
| Yes | 636 (66.1) | 326 (33.9) | 2.33 (2.06–2.63) | 946 (98.3) | 16 (1.7) | 1.32 (0.75–2.33) |
| Sex | ||||||
| Female | 1,759 (77.4) | 515 (22.6) | Ref. | 2,227 (97.9) | 47 (2.1) | Ref. |
| Male | 2,667 (86.5) | 418 (13.5) | 0.62 (0.55–0.70) | 3,056 (99.1) | 29 (0.9) | 0.50 (0.31–0.80) |
| Age (unit = 1 year) | 64 ± 11 | 66 ± 11 | 1.02 (1.01–1.03) | 64 ± 11 | 67 ± 10 | 1.02 (0.99–1.05) |
| Diabetes duration (unit = 1 year) | 4.8 ± 2.3 | 5.1 ± 2.5 | 1.03 (1.00–1.05) | 4.8 ± 2.3 | 5.1 ± 2.3 | 1.02 (0.95–1.10) |
| CCI | ||||||
| 0 | 2,564 (85.7) | 428 (14.3) | Ref. | 2,955 (98.8) | 37 (1.2) | Ref. |
| 1–2 | 1,418 (79.3) | 370 (20.7) | 1.26 (1.11–1.43) | 1,759 (98.4) | 29 (1.6) | 1.25 (0.77–2.05) |
| ≥3 | 444 (76.7) | 135 (23.3) | 1.29 (1.07–1.54) | 569 (98.3) | 10 (1.7) | 1.15 (0.51–2.58) |
| BMI (unit = 1 kg/m2) | 30.4 ± 5.8 | 31.0 ± 6.7 | 1.01 (1.00–1.02) | 30.5 ± 5.9 | 29.4 ± 7.3 | 0.97 (0.92–1.03) |
| Units of alcohol/week (F/M) | ||||||
| ≤7/14 | 3,674 (82.7) | 770 (17.3) | Ref. | 4,380 (98.6) | 64 (1.4) | Ref. |
| >7/14 | 697 (83.3) § | 140 (16.7) § | 1.07 (0.90–1.26) | 826 (98.7) | 11 (1.3) | 1.09 (0.56–2.12) |
| Current and former history of daily smoking | ||||||
| No | 1,577 (83.0) | 324 (17.0) | Ref. | 1,870 (98.4) | 31 (1.6) | Ref. |
| Yes | 2,837 (82.5) § | 603 (17.5) § | 0.99 (0.87–1.12) | 3,396 (98.7) | 44 (1.3) | 0.82 (0.50–1.33) |
| Eye disease | ||||||
| No | 3,810 (83.6) | 746 (16.4) | Ref. | 4,497 (98.7) | 59 (1.3) | Ref. |
| Yes | 616 (76.7) | 187 (23.3) | 1.15 (0.98–1.33) | 786 (97.9) | 17 (2.1) | 1.28 (0.70–2.32) |
Adjusted for all variables listed in the table: age, sex, body mass index (BMI), smoking, alcohol consumption, diabetes duration, Charlson Comorbidity Index (CCI) and eye disease. Confounder effect estimates were mutually adjusted.
aPR, adjusted prevalence ratio; CI, confidence interval; F, female; M, male; Ref., reference.
Possible diabetic polyneuropathy (DPN) defined as Michigan Neuropathy Screening Instrument Questionnaire score ≥4.
Data are presented as frequencies and percentages (n [%]) or as the mean ± standard deviation.
All analyses were carried out as complete case analyses (n = 5,178), as there were only a few missing variables. Missing data: possible DPN: 0; sex: 0; age: 0; diabetes duration: 2 (0.0%); CCI: 0; BMI: 93 (1.7%); alcohol: 78 (1.5%); smoking: 18 (0.3%); eye disease: 0.
Table 2.
Adjusted prevalence ratio of falls in type 2 diabetes patients with possible diabetic polyneuropathy compared with patients without possible diabetic polyneuropathy by the number of falls
| No. falls | ||||||||
|---|---|---|---|---|---|---|---|---|
|
No falls n (%) ‡ |
No falls aPR (95% CI) § |
1 Fall n (%) ‡ |
1 Fall aPR (95% CI) § |
2–4 falls n (%) ‡ |
2–4 Falls aPR (95% CI) § |
>4 Falls n (%) ‡ |
>4 Falls aPR (95% CI) § |
|
| Total | 4,426 (82.6) | – | 431 (8.0) | – | 393 (7.3) | – | 96 (1.8) | – |
| Possible DPN † | ||||||||
| No | 3,790 (86.2) | Ref. | 323 (7.3) | Ref. | 233 (5.3) | Ref. | 40 (0.9) | Ref. |
| Yes | 636 (66.1) | 0.78 (0.74–0.82) | 108 (11.2) | 1.51 (1.22–1.89) | 160 (16.6) | 2.86 (2.32–3.52) | 56 (5.8) | 5.89 (3.84–9.05) |
Adjusted for: age, sex, body mass index, smoking, alcohol consumption, diabetes duration, Charlson Comorbidity Index and eye disease.
aPR, adjusted prevalence ratio; CI, confidence interval; Ref., reference.
Diabetic polyneuropathy (DPN) defined as Michigan Neuropathy Screening Instrument Questionnaire score ≥ 4.
Data are presented as frequencies and percentages (n [%]) or as mean ± standard deviation.
All analyses were carried out as complete case analyses (n = 5,178), as there were only a few missing variables. Individuals with missing data: possible DPN: 0; sex: 0; age: 0; diabetes duration: 2 (0.0%); Charlson Comorbidity Index: 0; body mass index: 93 (1.7%); alcohol: 78 (1.5%); smoking: 18 (0.3%); eye disease: 0) and 13 patients (0.2%) did not specify the number of falls.
Among the 933 patients with at least one fall, 36% (n = 336) reported a subsequent contact with their general practitioner and/or a hospital. Patients without DPN sought medical attention to a higher degree than those with DPN, mainly driven by those reporting only one fall (Figure 2).
Figure 2.
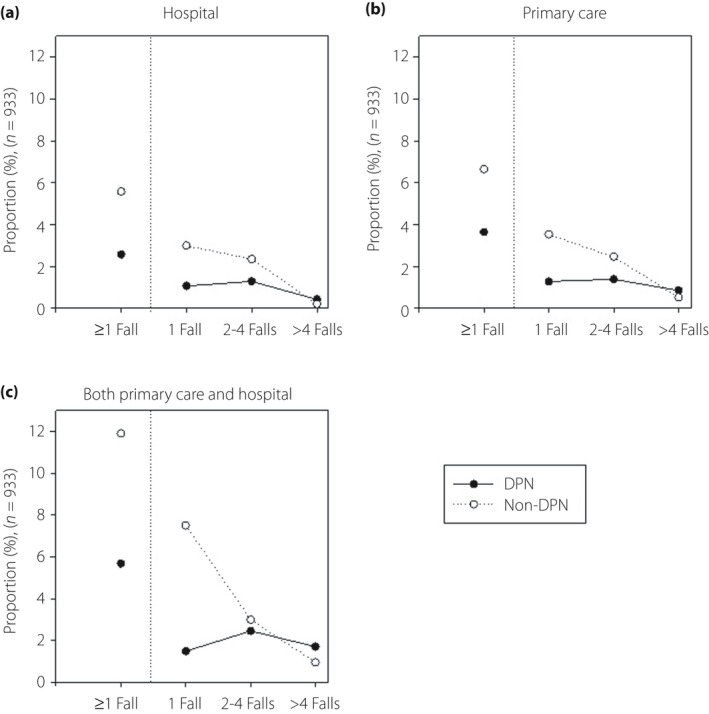
Proportion of patients with one or more falls (n = 933) who sought medical attention at hospitals, in primary care or both in primary care and hospitals. Filled circles, patients with possible diabetic polyneuropathy (DPN); empty circles: Patients without DPN.
DPN and fractures
In the year preceding DPN assessment, a total of 87 fractures were identified in 76 patients. Of these 87 fractures, 14 (16%) were fractures of the shoulder and upper arm; 14 (16%) of the forearm; 17 (20%) of the wrist and hand; 13 (15%) of the hip and other femoral fractures; 12 (14%) of the lower leg including ankle; eight (9%) fractures of the foot and toe, except ankle; five (6%) spinal fractures; one (1%) pelvic fracture; one (1%) of the skull and facial bones; and two (2%) fractures of the ribs and sternum (Table S5).
Possible DPN was associated with a slightly, although non‐significantly, increased risk of fractures (aPR 1.32, 95% CI 0.75–2.33) after adjusting for possible confounders (Table 1). Analyses stratified by sex showed similar results, although these findings were limited by low statistical power (Table S3). In the sensitivity analysis, extending the fracture assessment time period further attenuated the association (aPR 1.19, 95% CI 0.77–1.85; Table S6). Adding the use of antihypertensive medication use and insulin use to the regression model did not change the estimate (aPR 1.30, 95% CI 0.74–2.30; Table S4).
Other characteristics
Based on the confounder effect estimates, the following clinical characteristics had a positive correlation indicating confounding, when calculating prevalence ratios for falls in patients with DPN: comorbidity burden (CCI 1–2 vs 0, aPR 1.26, 95% CI 1.11–1.43 and CCI ≥3 vs 0, aPR 1.29, 95% CI 1.07–1.54), higher BMI (per 1‐kg/m2 increase, aPR 1.01, 95% CI 1.00–1.02), higher age (per 1‐year increase, aPR 1.02, 95% CI 1.01–1.03) and longer diabetes duration (per 1‐year increase, aPR 1.03, 95% CI 1.00–1.05; Table 1). Whereas male sex had a negative correlation, indicating confounding when calculating prevalence ratios for patients with DPN compared with patients without DPN both for falls and fractures (aPR 0.62, 95% CI 0.55–0.70 and aPR 0.50, 95% CI 0.31–0.80, respectively).
Discussion
In this large, nationwide, cross‐sectional, questionnaire study of patients with recently diagnosed type 2 diabetes, we determined the prevalence of falls and fractures, and evaluated the association with possible DPN based on the MNSIq.
Our main findings were that patients with recently diagnosed type 2 diabetes and possible DPN had a 2.3‐fold higher risk of falling compared with those without DPN, and the association gradually increased with a higher number of falls. DPN was associated with a slightly, although non‐significantly, increased risk of fractures. One‐third sought medical attention after a fall episode, which might indicate that these falls were severe and injurious. Noteworthy, seeking medical attention seemed less frequent in those with DPN compared with those without DPN, indicating that patients with DPN suffered less injurious falls.
The present findings show that possible DPN is strongly associated with falls in patients with recently diagnosed type 2 diabetes. This is the first study evaluating the association between symptoms of neuropathy and the incidence of falls and fractures by applying validated tools for the assessment of DPN. Our findings corroborate previous studies reporting an association between impaired vibration perception 32 , reduced nerve conduction velocities of the peroneal nerve 33 , and insensate feet 34 and increased risk of falls in patients with longer type 2 diabetes duration. Other factors that have been associated with falls in diabetes patients, include older age, female sex, increased BMI, polypharmacy, hypoglycemic episodes, insulin use, and macro‐ and microvascular complications 33 , 35 . However, adjustment for multiple confounders in the present study did not change the association between falls and possible DPN.
We found that fractures of upper and lower limbs were the most commonly reported fracture sites, and these findings are in agreement with other studies in type 2 diabetes patients 36 , 37 . In the present study, DPN was only slightly and non‐significantly associated to a higher prevalence of fractures. Previous studies showed that patients with longer type 2 diabetes duration have an increased risk of suffering from a fracture 37 , 38 , 39 when compared with healthy individuals. Higher bone fragility is suggested to be the cause of fractures in chronic type 2 diabetes 38 , which can occur due to accumulation of advanced glycation end‐products in bone collagen, increased urinary calcium excretion due to high blood glucose levels, microvascular damage and decreased bone turnover 40 , 41 . However, it remains unknown why patients with type 2 diabetes have a higher risk of suffering from fractures. Our population is characterized by a relatively high BMI, young age and relatively short diabetes duration, all of which are known to protect against bone fractures 38 ; however, adjusting for these potential confounders did not change the estimates. We identified fractures based on diagnosis codes; thus, we can only infer that they were fall‐related. However, the most common fracture sites were upper and lower limbs, including the hips, which are commonly injured in fall episodes, thus indicating that patients were “catching themselves from falling.”
In the present study, a significant fraction of patients reported seeking medical attention due to falling, whereas the number of fractures was relatively small. This suggests that although falling does not necessarily result in a bone fracture, there are many other severe and injurious consequences that require medical attention. After a fall, patients without DPN did seek medical attention more frequently than those with DPN, indicating that even though patients with DPN fall more often, the falls might be less severe. The high number of patients seeking medical attention in the present study is worrisome, as our population was younger and had a shorter duration of diabetes compared with other studies describing similar associations. We did not obtain data on the types of injuries other than fractures.
Falls were self‐reported and recorded retrospectively, which might introduce a possible recall bias or a misinterpretation of the definition of a fall by patients. Furthermore, the cross‐sectional design of the study did not allow for a temporal assessment. In the present study, multiple confounders were taken into consideration. Medications, such as benzodiazepines or opiates, might increase the risk of falling. However, as these medications might also be prescribed due to fracture occurrence and for the treatment of neuropathic pain and sleep disorders, these medications might be a part of the causal pathway and thereby not solely considered as confounders. Therefore, we did not adjust for these medications in the present study. Hyperglycemic status might be associated with increased fracture risk 42 . We did not adjust for glycated hemoglobin (HbA1c), as we obtained data on HbA1c in a minor subpopulation only; thus, we did not include HbA1c in the regression analysis. However, we did include both diabetes duration and comorbidities in the regression model, which might to some degree provide similar information as Hba1c levels. Furthermore, we attained data on insulin use, which might be an indicator of the severity in dysregulated diabetes, and a marker of increased risk of hypoglycemia. Adding insulin use to the regression model did not attenuate the impact of DPN. Because of a low number of total fractures and limitations in the specificity of International Classification of Diseases, version 10 coding and the fact that the DNPR does not distinguish clearly between osteoporotic and non‐osteoporotic fractures, we could not examine osteoporotic and non‐osteoporotic fractures separately. This should be considered in future studies.
We used a validated questionnaire to determine the presence of DPN. However, as our DPN diagnosis did not include clinical evaluations and neurophysiological examinations, the diagnosis of DPN is only at the level of ‘possible’ DPN according to the Toronto Classification of DPN 11 . The MNSIq has a fairly low sensitivity (40%) and high specificity (92%) 43 , which is more important when carrying out measures of relative risk, as described in detail elsewhere 44 . As of today, there are still no easily assessable tools available for screening and early identification of type 2 diabetes patients at risk of falling. Interestingly, we found that even in recently diagnosed diabetes, patients report considerably more falls when having symptoms of DPN. The present study can aid in the development of future fall prevention programs and the identification of patients at risk; however, this needs to be further studied in large‐scale prospective studies.
In summary, we found that patients with recently diagnosed type 2 diabetes and possible DPN were 2.3‐fold more likely to have suffered from a fall than those without possible DPN, whereas possible DPN was only slightly and non‐significantly associated with fractures. Identifying patients with possible DPN might help in detecting patients at risk of falling and should be considered in future longitudinal studies on fall prevention.
Disclosure
The authors declare no conflicts of interest. Research reported in this publication is part of the International Diabetic Neuropathy Consortium (IDNC) research program, which is supported by a Novo Nordisk Foundation Challenge Program grant (grant number NNF14OC0011633) and Aarhus University. The Danish Center for Strategic Research in Type 2 Diabetes Project (DD2) is supported by the Danish Agency for Science (grant number 09‐067009, 09‐075724), the Danish Health and Medicines Authority, the Danish Diabetes Association, and an unrestricted donation from Novo Nordisk A/S. Project partners are listed on the website www.DD2.nu. The Department of Clinical Epidemiology, Aarhus University Hospital, receives funding for other studies from companies in the form of research grants to (and administered by) Aarhus University. None of these studies had any relation to the present study.
Supporting information
Figure S1 | Flowchart of study population.
Table S1 | International Classification of Diseases, version 10 codes used for data extraction regarding fractures from the Danish National Patient Register.
Table S2 | International Classification of Diseases, version 10 and International Classification of Diseases, version8 codes used for the data extraction and estimation of the Charlson Comorbidity Index from the Danish National Patient Register.
Table S3 | Adjusted prevalence ratio of falls and fractures in patients with possible DPN compared to patients without possible diabetic polyneuropathy. Stratified analyses according to biological sex.
Table S4 | Adjusted prevalence ratio of fractures in patients with possible diabetic polyneuropathy compared with patients without possible diabetic polyneuropathy (more extensive adjustment including antihypertensive medication and insulin).
Table S5 | Total number of fractures extracted from the Danish National Patient Register (7 June 2015 to 10 October 2016).
Table S6 | Adjusted prevalence ratio of fractures in patients with possible diabetic polyneuropathy compared with patients without possible diabetic polyneuropathy (sensitivity analysis for the entire time period from 7 June 2015 to 24 January 2017).
J Diabetes Investig. 2021; 12: 1827–1834
References
- 1. Vinik AI, Vinik EJ, Colberg SR, et al. Falls risk in older adults with type 2 diabetes. Clin Geriatr Med 2015; 31: 89–99, viii. [DOI] [PubMed] [Google Scholar]
- 2. Yang Y, Hu X, Zhang Q, et al. Diabetes mellitus and risk of falls in older adults: a systematic review and meta‐analysis. Age Ageing 2016; 45: 761–767. [DOI] [PubMed] [Google Scholar]
- 3. Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged ≥65 years – United States, 2014. MMWR Morb Mortal Wkly Rep 2016; 65: 993–998. [DOI] [PubMed] [Google Scholar]
- 4. Tinetti ME. Preventing falls in elderly persons. N Engl J Med 2003; 348: 42–49. [DOI] [PubMed] [Google Scholar]
- 5. Health M, Section E, Pijpers E, et al. Older individuals with diabetes have an increased risk of recurrent falls: analysis of potential mediating factors : the Longitudinal Ageing Study Amsterdam. Age Ageing 2012; 30: 358–365. [DOI] [PubMed] [Google Scholar]
- 6. Richardson JK, Hurvitz EA. Peripheral neuropathy: a true risk factor for falls. J Gerontol A Biol Sci Med Sci 1995; 50: M211–M215. [DOI] [PubMed] [Google Scholar]
- 7. Schwartz AV. Epidemiology of fractures in type 2 diabetes. Bone 2016; 82: 2–8. [DOI] [PubMed] [Google Scholar]
- 8. Yau RK, Strotmeyer ES, Resnick HE, et al. Diabetes and risk of hospitalized fall injury among older adults. Diabetes Care 2013; 36: 3985–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pscherer S, Sandmann GH, Ehnert S, et al. Delayed fracture healing in diabetics with distal radius fractures. Acta Chir Orthop Traumatol Cech 2017; 84: 24–29. [PubMed] [Google Scholar]
- 10. Enomoto LM, Shrestha DP, Rosenthal MB, et al. Risk factors associated with 30‐day readmission and length of stay in patients with type 2 diabetes. J Diabetes Complications 2017; 31: 122–127. [DOI] [PubMed] [Google Scholar]
- 11. Tesfaye S, Boulton AJM, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010; 33: 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Simoneau GG, Ulbrecht JS, Derr JA, et al. Postural instability in patients with diabetic sensory neuropathy. Diabetes Care 1994; 17: 1411–1421. [DOI] [PubMed] [Google Scholar]
- 13. Alam U, Riley DR, Jugdey RS, et al. Diabetic neuropathy and gait: a review. Diabetes Ther. 2017; 8: 1253–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Timar B, Timar R, Gaiță L, et al. The impact of diabetic neuropathy on balance and on the risk of falls in patients with type 2 diabetes mellitus: a cross‐sectional study. PLoS One 2016; 11: e0154654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andersen H, Nielsen S, Mogensen CE, et al. Muscle strength in type 2 diabetes. Diabetes 2004; 53: 1543–1548. [DOI] [PubMed] [Google Scholar]
- 16. Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care 2002; 25: 1749–1754. [DOI] [PubMed] [Google Scholar]
- 17. Bokan‐Mirković V, Škarić‐Karanikić Ž, Nejkov S, et al. Diabetic polyneuropathy and risk of falls: fear of falling and other factors. Acta Clin Croat 2017; 56: 721–727. [DOI] [PubMed] [Google Scholar]
- 18. Roman de Mettelinge T, Cambier D, Calders P, et al. Understanding the relationship between type 2 diabetes mellitus and falls in older adults: a prospective cohort study. Bayer A, editor. PLoS One 2013; 8: e67055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. MacGilchrist C, Paul L, Ellis BM, et al. Lower‐limb risk factors for falls in people with diabetes mellitus. Diabet Med 2010; 27: 162–168. [DOI] [PubMed] [Google Scholar]
- 20. Lee RH, Sloane R, Pieper C, et al. Clinical fractures among older men with diabetes are mediated by diabetic complications. J Clin Endocrinol Metab 2018; 103: 281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim J‐H, Jung M‐H, Lee J‐M, et al. Diabetic peripheral neuropathy is highly associated with nontraumatic fractures in Korean patients with type 2 diabetes mellitus. Clin Endocrinol (Oxf) 2012; 77: 51–55. [DOI] [PubMed] [Google Scholar]
- 22. Melton LJ, Leibson CL, Achenbach SJ, et al. Fracture risk in type 2 diabetes: update of a population‐based study. J Bone Miner Res 2008; 23: 1334–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chau RMW, Ng TKW, Kwan RLC, et al. Risk of fall for people with diabetes. Disabil Rehabil 2013; 35: 1975–1980. [DOI] [PubMed] [Google Scholar]
- 24. Patel S, Hyer S, Tweed K, et al. Risk factors for fractures and falls in older women with type 2 diabetes mellitus. Calcif Tissue Int 2008; 82: 87–91. [DOI] [PubMed] [Google Scholar]
- 25. Yokomoto‐Umakoshi M, Kanazawa I, Kondo S, et al. Association between the risk of falls and osteoporotic fractures in patients with type 2 diabetes mellitus. Endocr J 2017; 64: 727–734. [DOI] [PubMed] [Google Scholar]
- 26. Nielsen JS, Thomsen R, Steffensen C, et al. The Danish Centre for Strategic Research in Type 2 Diabetes (DD2) study: implementation of a nationwide patient enrollment system. Clin Epidemiol 2012; 4(Suppl 1): 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Christensen DH, Nicolaisen SK, Berencsi K, et al. Danish Centre for Strategic Research in Type 2 Diabetes (DD2) project cohort of newly diagnosed patients with type 2 diabetes: a cohort profile. BMJ Open 2018; 8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gylfadottir SS, Christensen DH, Nicolaisen SK, et al. Diabetic polyneuropathy and pain, prevalence, and patient characteristics. Pain 2020; 161: 574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Freiberger E, de Vreede P. Falls recall—limitations of the most used inclusion criteria. Eur Rev Aging Phys Act 2011; 8: 105–108. [Google Scholar]
- 30. Schmidt M, Schmidt SAJ, Sandegaard JL, et al. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015; 7: 449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 32. de Mettelinge TR, Calders P, Palmans T, et al. Vibration perception threshold in relation to postural control and fall risk assessment in elderly: disability vibration perception threshold in relation to postural control and fall risk assessment in elderly. Rehabil 2013; 35: 1712–1717. [DOI] [PubMed] [Google Scholar]
- 33. Schwartz AV, Vittinghoff E, Sellmeyer DE, et al. Diabetes‐related complications, glycemic control, and falls in older adults. Diabetes Care 2008; 31: 391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wallace C, Reiber GE, LeMaster J, et al. Incidence of falls, risk factors for falls, and fall‐related fractures in individuals with diabetes and a prior foot ulcer. Diabetes Care 2002; 25: 1983–1986. [DOI] [PubMed] [Google Scholar]
- 35. Herrera‐Rangel AB, Aranda‐Moreno C, Mantilla‐Ochoa T, et al. Influence of the body mass index on the occurrence of falls in patients with type 2 diabetes mellitus. Obes Res Clin Pract 2015; 9: 522–526. [DOI] [PubMed] [Google Scholar]
- 36. Vestergaard P, Rejnmark L, Mosekilde L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia 2005; 48: 1292–1299. [DOI] [PubMed] [Google Scholar]
- 37. Wallander M, Axelsson KF, Nilsson AG, et al. Type 2 diabetes and risk of hip fractures and non‐skeletal fall injuries in the elderly: a study from the fractures and fall injuries in the elderly cohort (FRAILCO). J Bone Miner Res 2017; 32: 449–460. [DOI] [PubMed] [Google Scholar]
- 38. Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta‐analysis. Osteoporos Int 2007; 18: 427–444. [DOI] [PubMed] [Google Scholar]
- 39. Moayeri A, Mohamadpour M, Mousavi S, et al. Fracture risk in patients with type 2 diabetes mellitus and possible risk factors: a systematic review and meta‐analysis. Ther Clin Risk Manag 2017; 13: 455–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saito M, Kida Y, Kato S, et al. Diabetes, collagen, and bone quality. Curr Osteoporos Rep 2014; 12: 181–188. [DOI] [PubMed] [Google Scholar]
- 41. Carulli C, Innocenti M, Brandi ML. Bone vascularization in normal and disease conditions. Front Endocrinol 2013; 4: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hygum K, Starup‐Linde J, Langdahl BL. Diabetes and bone. Osteoporos Sarcopenia 2019; 5: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Herman WH, Pop‐Busui R, Braffett BH, et al. Use of the Michigan neuropathy screening instrument as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the diabetes control and complications trial/epidemiology of diabetes interventions and complications. Diabet Med 2012; 29: 937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Christensen DH, Knudsen ST, Gylfadottir SS, et al. Metabolic factors, lifestyle habits, and possible polyneuropathy in early type 2 diabetes: a nationwide study of 5,249 patients in the Danish centre for strategic research in type 2 diabetes (DD2) cohort. Diabetes Care 2020; 43: 1266–1275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Flowchart of study population.
Table S1 | International Classification of Diseases, version 10 codes used for data extraction regarding fractures from the Danish National Patient Register.
Table S2 | International Classification of Diseases, version 10 and International Classification of Diseases, version8 codes used for the data extraction and estimation of the Charlson Comorbidity Index from the Danish National Patient Register.
Table S3 | Adjusted prevalence ratio of falls and fractures in patients with possible DPN compared to patients without possible diabetic polyneuropathy. Stratified analyses according to biological sex.
Table S4 | Adjusted prevalence ratio of fractures in patients with possible diabetic polyneuropathy compared with patients without possible diabetic polyneuropathy (more extensive adjustment including antihypertensive medication and insulin).
Table S5 | Total number of fractures extracted from the Danish National Patient Register (7 June 2015 to 10 October 2016).
Table S6 | Adjusted prevalence ratio of fractures in patients with possible diabetic polyneuropathy compared with patients without possible diabetic polyneuropathy (sensitivity analysis for the entire time period from 7 June 2015 to 24 January 2017).


