Abstract
Introduction
Fatty liver disease (FLD) is a surrogate condition for glucose intolerance development. FLD may involve normal or abnormal liver enzyme levels. Whether FLD is a risk factor for glucose intolerance, regardless of liver enzyme levels, remains unknown. We assessed relationships between the development of impaired fasting glucose (IFG) and FLD, liver enzyme abnormalities, and alcohol consumption.
Materials and Methods
We retrospectively evaluated 8,664 participants with more than two annual health check‐ups. Participants were classified according to sex, alcohol consumption, alanine aminotransferase (ALT) levels, and fatty liver status.
Results
In univariate analyses, IFG onset among men was related to normal or high ALT levels with FLD in the nonalcoholic and alcoholic groups (P‐trend < 0.01). In multivariate analyses, IFG onset among nonalcoholic men was associated with normal or high ALT levels with FLD, independent of potential confounding factors (P‐trend < 0.01). However, IFG onset was non‐independently associated with any condition among alcoholic men. In univariate analyses, IFG onset among women was related to normal or high ALT levels with FLD in the nonalcoholic group (P‐trend < 0.01) and high ALT levels with FLD in the alcoholic group (P‐trend < 0.05). In multivariate analyses, IFG onset was independently associated with only normal ALT levels in nonalcoholic FLD women.
Conclusions
Among nonalcoholic men and women, FLD was a risk factor for IFG onset, including normal ALT concentrations. Care is needed for individuals with nonalcoholic FLD, regardless of liver injury, possibly helping reduce glucose intolerance risk.
Keywords: Abnormal glucose tolerance, Fatty liver, Normal alanine aminotransferase
Whether fatty liver disease is a risk factor for glucose intolerance onset, regardless of liver enzyme levels, remains unknown. Among nonalcoholic Japanese people, fatty liver was related to impaired fasting glucose onset, even with normal alanine aminotransferase levels.

INTRODUCTION
The increase in the incidence of metabolic diseases is linked to relatively recent lifestyle changes, such as poor diet and lack of exercise. Among these metabolic diseases, diabetes significantly increases the risk of serious progressive diseases such as polyneuropathy, renal failure, visual loss, cardiovascular disease, and hepatocellular carcinoma 1 , 2 , 3 , 4 . Therefore, it is important to not only prevent the onset of diabetes but also treat patients who already have diabetes.
Several reports have described markers for prediabetes that may help guide interventions to prevent the onset of diabetes. Fatty liver disease (FLD) is one of the most efficient surrogate conditions for identifying patients with an elevated risk of developing prediabetes and diabetes 5 , 6 , 7 . In this context, FLD is mainly categorized as nonalcoholic FLD (NAFLD) and alcoholic FLD (AFLD). NAFLD is clearly linked to the onset and presence of glucose intolerance 8 , 9 , 10 , although an AFLD is not a clear risk factor for the development of glucose intolerance 11 , 12 , 13 , 14 . However, some patients with FLDs have normal liver enzyme levels, while in others, the levels are abnormal, and it remains unclear whether NAFLD or AFLD, regardless of the liver enzyme levels, is a risk factor for abnormal glucose tolerance. Therefore, we retrospectively assessed the relationships between the development of impaired fasting glucose (IFG) and FLD, liver enzyme abnormalities, and alcohol consumption.
MATERIALS AND METHODS
Participants
This retrospective study evaluated the medical records of 9,817 community‐based Japanese individuals (4,793 men and 5,024 women, age: 21–78 years) who had undergone more than two annual health check‐ups at the Ehime General Health Care Association between April 2003 and March 2017. The study protocol was approved by the Committee for Medical Ethics of Ehime University Hospital (approval no.: 1709007, University Hospital Medical Information Network ID: UMIN000016379) and was conducted according to the tenets of the Declaration of Helsinki and its later amendments. All participants were assigned numerical codes to protect their identity and the anonymized records were stored in a secure database. Informed consent was not required due to the study being a retrospective analysis of anonymized data.
After evaluating the laboratory data and medical background from the first check‐up, 1,153 participants were excluded for the following reasons: (a) currently receiving anti‐diabetic agents (n = 131), (b) baseline FPG of ≥ 6.11 mM (n = 698), (c) alcoholic status plus normal ALT levels but without FLD (n = 131), and/or (d) positivity for HBsAg (n = 131) or anti‐HCV antibody (n = 98) (Figure 1). Thus, a total of 8,664 participants were considered eligible (3,964 men and 4,700 women) and categorized into five groups, with each group further categorized according to sex: normal (men: n = 2,106, women: n = 3,588), nonalcoholic normal ALT levels with FLD (men: n = 551, women: n = 280), nonalcoholic high ALT levels with FLD (men: n = 715, women: n = 235), nonalcoholic abnormal ALT levels and non‐fatty liver disease (non‐FLD) (men: n = 389, women: n = 538), alcoholic normal ALT levels with FLD (men: n = 71, women: n = 15), alcoholic high ALT levels with FLD (men: n = 68, women: n = 11), and alcoholic abnormal ALT levels and non‐FLD (men: n = 64, women: n = 33). The average observation period was 5.61 ± 3.52 years (men: 5.42 ± 3.52 years, women: 5.78 ± 3.51 years).
Figure 1.
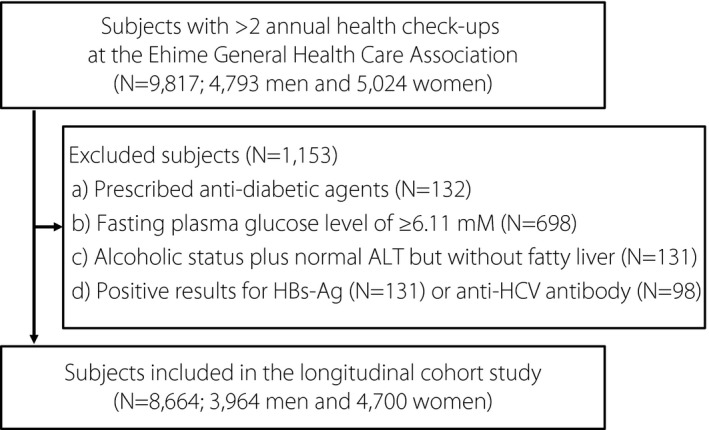
Flowchart of the study participant selection process. HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus.
Examinations
The participants underwent physical examination and routine testing to evaluate biochemical factors. Height and body weight of the participants were assessed with light gowns on, without shoes, and the results were used to calculate the body mass index (BMI). Blood pressure was measured using an automated sphygmomanometer while the participants were in the sitting position. Blood samples were acquired in the morning after a ≥ 10‐h fast and were used to determine the values for fasting plasma glucose (FPG), hemoglobin A1c (HbA1c), aspartate aminotransferase (AST), ALT, serum creatinine (Cre), uric acid (UA), total cholesterol (TC), triglycerides (TG), hepatitis B surface antigen (HBsAg), and antibodies for the hepatitis C virus (anti‐HCV antibody).
Before the health check‐up, public health nurses examined the questionnaires for their medical history, prescribed medications, family history of diabetes within two relatives, exercise habits (no habit or awareness of exercise vs. periodic exercise), snacking (no snacking vs. snacking ≥ 1 time/day), and average frequencies and quantities of alcohol consumption for all the participants 15 .
Patients were diagnosed with FLD using abdominal ultrasonography by trained technicians who did not know the patients’ data. The diagnosis of FLD was made depending on two types of evidence (hepatorenal contrast and liver brightness) and four different criteria (hepatorenal echo contrast, liver brightness, deep attenuation, and vascular blurring) 16 , 17 .
Definitions
Baseline ALT levels were defined as “normal” (men: ≤30 U/L, women: ≤20 U/L) or “high” (men: >30 U/L, women: >20 U/L) 18 . Alcohol consumption was categorized as “nonalcoholic” (men: no drinking or < 210 g/week, women: no drinking or < 140 g/week) or “alcoholic” (men: ≥210 g/week, women: ≥140 g/week) (Table S1 for men and Table S2 for women). IFG onset within the observation period was assessed as an FPG level of ≥ 6.11 mM at each health check‐up 19 , 20 . The reference group (“normal group”) was defined as nonalcoholic with a normal ALT levels and without FLD.
Statistical analysis
For all statistical analyses, we used JMP software for Windows (version 14.2; SAS Institute Japan, Tokyo, Japan). Normality assumption was tested using Kolmogorov‐Smirnov‐Lilliefors test, which revealed that none of the continuous variables were normally distributed. Thus, inter‐group comparisons of continuous variables (i.e., age, BMI, blood pressure, and other routine biochemical variables) were performed using Mann–Whitney U test and Steel‐Dwass test. Chi‐square test was used to evaluate categorical variables, which included sex, baseline lifestyle habits, and onset of IFG. Statistically significant differences were considered if two‐tailed P‐values were < 0.05.
Univariate Cox proportional hazards regression analyses using the forward likelihood ratio test were performed to assess the variables’ relationship with the onset of IFG. Multivariate Cox proportional hazards regression analyses were performed to calculate the adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for developing IFG. The multivariate model was adjusted for the following baseline metabolic disease‐related factors: age, sex, BMI, systolic blood pressure (SBP), FPG, TC, TG, UA, Cre, exercise and snacking habits, and family history of diabetes 21 , 22 , 23 , 24 , 25 . The trend of association was assessed using a logistic regression model that assigned consecutive integers to the categories of the exposure variables. Spearman's correlation coefficients were used to evaluate the relationship between the ALT level at baseline and the ALT level at endpoint in participants with the onset of IFG.
RESULTS
Characteristics at baseline
Tables 1, 2, 3, 4 and Tables S3 and S4 show the participants’ baseline characteristics according to the category of group. Complete data were available for the vast majority of patients, although data were missing regarding the BMI (one subject), exercise habits (two participants), and snacking habits (two participants).
Table 1.
Baseline characteristics of nonalcoholic men
| Normal (n = 2,106) | Normal ALT levels and fatty liver disease (n = 551) | High ALT levels and fatty liver disease (n = 715) | P | |
|---|---|---|---|---|
| Age, years | 42 (35–50) | 45 (38–51) | 42 (36–47) | <0.01a,c |
| BMI, kg/m2 | 22.2 (20.7–23.8) | 24.8 (23.2–26.5) | 26.2 (24.3–28.3) | <0.01a,b,c |
| SBP, mmHg | 113 (104–123) | 119 (109–131) | 121 (112–134) | <0.05a,b,c |
| DBP, mmHg | 71 (64–79) | 75 (67–84) | 76 (69–85) | <0.05a,b,c |
| FPG, mM | 5.16 (4.94–5.44) | 5.33 (5.05–5.55) | 5.33 (5.05–5.55) | <0.01a,b |
| HbA1c, % | 5.4 (5.1–5.6) | 5.5 (5.3–5.7) | 5.6 (5.4–5.8) | <0.01a,b |
| AST, IU/L | 20 (17–22) | 20 (18–22) | 28 (25–35) | <0.01b,c |
| ALT, IU/L | 18 (15–23) | 22 (18–26) | 45 (36–60) | <0.01a,b,c |
| Cre, µM | 79.6 (70.7–83.1) | 79.6 (70.7–88.4) | 79.6 (70.7–86.6) | |
| UA, µM | 350.9 (315.2–398.5) | 380.7 (333.1–422.3) | 398.5 (356.9–446.1) | <0.01a,b,c |
| TC, mM | 5.07 (4.53–5.64) | 5.35 (4.81–5.95) | 5.4 (4.89–5.95) | <0.01a,b |
| TG, mM | 1.02 (0.76–1.39) | 1.48 (1.03–2.1) | 1.76 (1.24–2.46) | <0.01a,b,c |
| Periodic exercise*, n | 841 (39.9%) | 172 (31.3%) | 190 (26.6%) | <0.01 |
| Snacking habits**, n | 959 (45.6%) | 287 (52.1%) | 380 (53.2%) | <0.01 |
| Family history of diabetes, n | 321 (15.2%) | 91 (16.5%) | 120 (16.8%) | 0.542 |
| Onset of impaired fasting glucose***, n | 138 (6.6%) | 83 (15.1%) | 137 (19.2%) | <0.01 |
Data are presented as the median (interquartile range) or number (percentage). *Exercise habit: no habit or awareness of exercise vs. periodic exercise. **Snacking habit: no snacking vs. snacking ≥ 1 time/day. ***Onset of impaired fasting glucose: ≥6.11 mM during the observation period. The Steel‐Dwass test was used to analyze continuous variables, and the χ2 test was used to analyze categorical variables. Differences were considered significant at P < 0.05 (aNormal vs. Normal ALT levels and fatty liver disease; bNormal vs. High ALT levels and fatty liver disease; cNormal ALT levels and fatty liver disease vs. High ALT levels and fatty liver disease). AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; Cre, creatinine; UA, uric acid; TC, total cholesterol; TG, triglycerides.
Table 2.
Baseline characteristics of alcoholic men
| Normal (n = 2,106) | Normal ALT levels and fatty liver disease (n = 71) | High ALT levels and fatty liver disease (n = 68) | P | |
|---|---|---|---|---|
| Age, years | 42 (35–50) | 48 (41–54) | 45.5 (39–50.8) | <0.05a,b |
| BMI, kg/m2 | 22.2 (20.7–23.8) | 25 (23.1–27) | 26 (24.1–27.8) | <0.01a,b |
| SBP, mmHg | 113 (104–123) | 126 (116–138) | 130 (118–138) | <0.01a,b |
| DBP, mmHg | 71 (64–79) | 82 (74–91) | 83 (74–91.5) | <0.01b,c |
| FPG, mM | 5.16 (4.94–5.44) | 5.49 (5.33–5.77) | 5.38 (5.16–5.66) | <0.01a,b |
| HbA1c, % | 5.4 (5.1–5.6) | 5.6 (5.4–5.8) | 5.5 (5.2–5.7) | <0.01a |
| AST, IU/L | 20 (17–22) | 22 (18–25) | 34.5 (28–42.75) | <0.01a |
| ALT, IU/L | 18 (15–23) | 22 (18–27) | 44.5 (38.3–56.3) | <0.01a,b,c |
| Cre, µM | 79.6 (70.7–83.1) | 76 (67.2–79.6) | 70.7 (69.2–79.6) | <0.01b |
| UA, µM | 350.9 (315.2–398.5) | 380.7 (339–434.2) | 4.25.3 (386.6–462.5) | <0.01a,b,c |
| TC, mM | 5.07 (4.53–5.64) | 5.3 (4.97–5.97) | 5.56 (5.09–6.03) | <0.01a,b |
| TG, mM | 1.02 (0.76–1.39) | 1.76 (1.24–2.6) | 1.94 (1.25–2.69) | <0.01a,b |
| Periodic exercise*, n | 841 (39.9%) | 26 (36.6%) | 22 (32.4%) | 0.4 |
| Snacking habits**, n | 959 (45.6%) | 12 (16.9%) | 16 (23.5%) | <0.01 |
| Family history of diabetes, % | 321 (15.2%) | 15 (21.1%) | 17 (25%) | 0.04 |
| Onset of impaired fasting glucose***, n | 138 (6.6%) | 11 (15.5%) | 14 (20.6%) | <0.01 |
Data are presented as the median (interquartile range) or number (percentage). *Exercise habit: no habit or awareness of exercise vs. periodic exercise. **Snacking habit: no snacking vs. snacking ≥ 1 time/day. ***Onset of impaired fasting glucose: ≥6.11 mM during the observation period. The Steel‐Dwass test was used to analyze continuous variables, and the χ2 test was used to analyze categorical variables. Differences were considered significant at P < 0.05 (aNormal vs. Normal ALT levels and fatty liver disease; bNormal vs. High ALT levels and fatty liver disease; cNormal ALT levels and fatty liver disease vs. High ALT levels and fatty liver disease). AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; Cre, creatinine; UA, uric acid; TC, total cholesterol; TG, triglycerides.
Table 3.
Baseline characteristics of nonalcoholic women
| Normal group (n = 3,588) | Normal ALT levels and fatty liver disease (n = 280) | High ALT levels and fatty liver disease (n = 235) | P | |
|---|---|---|---|---|
| Age, years | 40 (34–46) | 44 (37–50) | 49 (42–54) | <0.01a,b,c |
| BMI, kg/m2 | 20.4 (19–22.1) | 24.1 (21.8–26.5) | 26.6 (23.8–29.1) | <0.01a,b,c |
| SBP, mmHg | 104 (96–114) | 115 (105–128) | 119 (109–134) | <0.05a,b,c |
| DBP, mmHg | 64 (58–71) | 70 (63–79) | 75 (66–82) | <0.01a,b,c |
| FPG, mM | 4.88 (4.61–5.11) | 5.08 (4.88–5.38) | 5.27 (5–5.61) | <0.01a,b,c |
| HbA1c, % | 5.4 (5.2–5.6) | 5.6 (5.4–5.8) | 5.7 (5.5–5.9) | <0.01a,b,c |
| AST, IU/L | 18 (16–20) | 18 (16–20) | 25 (21–30) | <0.01b,c |
| ALT, IU/L | 13 (11–16) | 15 (13–18) | 30 (24–42) | <0.01a,b,c |
| Cre, µM | 53 (53–61.9) | 53 (53–61.9) | 53 (51.3–61.9) | <0.05b |
| UA, µM | 249.8 (214.1–279.6) | 273.6 (232–309.3) | 309.3 (267.7–350.9) | <0.01a,b,c |
| TC, mM | 5.09 (4.55–5.69) | 5.35 (4.82–5.89) | 5.74 (5.2–6.26) | <0.01a,b,c |
| TG, mM | 0.69 (0.54–0.93) | 0.97 (0.69–1.42) | 1.37 (1.01–1.91) | <0.01a,b,c |
| Periodic exercise*, n | 952 (26.5%) | 66 (23.7%) | 49 (20.9%) | 0.1 |
| Snacking habits**, n | 3,105 (86.5%) | 248 (88.6%) | 208 (88.5%) | 0.45 |
| Family history of diabetes, N | 758 (21.1%) | 71 (25.4%) | 74 (31.5%) | <0.01 |
| Onset of impaired fasting glucose***, n | 80 (2.2%) | 26 (9.3%) | 35 (14.9%) | <0.01 |
Data are presented as the median (interquartile range) or umber (percentage). *Exercise habit: no habit or awareness of exercise vs. periodic exercise. **Snacking habit: no snacking vs. snacking ≥ 1 time/day. ***Onset of impaired fasting glucose: ≥6.11 mM during the observation period. The Steel‐Dwass test was used to analyze continuous variables, and the χ2 test was used to analyze categorical variables. Differences were considered significant at P < 0.05 (a Normal vs. Normal ALT levels and fatty liver disease; b Normal vs. High ALT levels and fatty liver disease; c Normal ALT levels and fatty liver disease vs. High ALT levels and fatty liver disease). AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; Cre, creatinine; UA, uric acid; TC, total cholesterol; TG, triglycerides.
Table 4.
Baseline characteristics of alcoholic women
| Normal (n = 3,588) | Normal ALT levels and fatty liver disease (n = 15) | High ALT levels and fatty liver disease (n = 11) | P | |
|---|---|---|---|---|
| Age, years | 40 (34–46) | 44 (39–49) | 47 (45–53) | <0.01b |
| BMI, kg/m2 | 20.4 (19–22.1) | 24.6 (22.3–28.5) | 27 (24–29.7) | <0.01a,b |
| SBP, mmHg | 104 (96–114) | 115 (106–125) | 129 (116–144) | <0.05a,b |
| DBP, mmHg | 64 (58–71) | 73 (65–80) | 84 (80–86) | <0.05a,b |
| FPG, mM | 4.88 (4.61–5.11) | 5.05 (4.88–5.38) | 5.32 (5.16–5.55) | <0.05a,b |
| HbA1c, % | 5.4 (5.2–5.6) | 5.6 (5.4–5.8) | 5.6 (5.4–5.9) | <0.05a |
| AST, IU/L | 18 (16–20) | 19 (17–21) | 25 (23–29) | <0.01b,c |
| ALT, IU/L | 13 (11–16) | 15 (11–17) | 25 (22–30) | <0.05b,c |
| Cre, µM | 53 (53–61.9) | 53 (49.5–61.9) | 53 (44.2–61.9) | |
| UA, µM | 249.8 (214.1–279.6) | 279.6 (261.7–350.9) | 327.1 (267.7–356.9) | <0.05a,b |
| TC, mM | 5.09 (4.55–5.69) | 4.73 (4.42–5.45) | 5.66 (5.4–6.18) | <0.05b,c |
| TG, mM | 0.69 (0.54–0.93) | 1.05 (0.52–1.53) | 1.47 (1.15–1.86) | <0.05b |
| Periodic exercise*, n | 952 (26.5%) | 5 (33.3%) | 2 (18.2%) | 0.69 |
| Snacking habits**, n | 3,105 (86.5%) | 9 (60%) | 6 (54.6%) | <0.01 |
| Family history of diabetes, N | 758 (21.1%) | 7 (46.7%) | 3 (27.3%) | 0.048 |
| Onset of impaired fasting glucose***, n | 80 (2.2%) | 1 (6.7%) | 1 (9.1%) | 0.16 |
Data are presented as the median (interquartile range) or number (percentage). *Exercise habit: no habit or awareness of exercise vs. periodic exercise. **Snacking habit: no snacking vs. snacking ≥ 1 time/day. ***Onset of impaired fasting glucose: ≥6.11 mM during the observation period. The Steel‐Dwass test was used to analyze continuous variables, and the χ2 test was used to analyze categorical variables. Differences were considered significant at P < 0.05 (aNormal vs. Normal ALT levels and fatty liver disease; bNormal vs. High ALT levels and fatty liver disease; cNormal ALT levels and fatty liver disease vs. High ALT levels and fatty liver disease). AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; Cre, creatinine; UA, uric acid; TC, total cholesterol; TG, triglycerides.
Among nonalcoholic men, and relative to the normal group, the groups that had normal ALT levels with FLD, especially high ALT levels with FLD, and abnormal ALT levels without FLD had significantly higher values for many metabolic markers such as BMI, SBP, DBP, FPG, HbA1c, AST, UA, TC, TG, and IFG onset rate (Table 1 and Table S3). Relative to the normal group, lower exercise rates were observed in the groups that had normal ALT levels with FLD, and high ALT levels with FLD, and abnormal ALT levels without FLD (Table 1 and Table S3). Furthermore, relative to the normal group, high rates of snacking were observed in the groups that had normal ALT levels with FLD and high ALT levels with FLD (Table 1). The metabolic markers were similar between the alcoholic and nonalcoholic groups of men (Table 2 and Table S4).
Among nonalcoholic women, and relative to the normal group, many metabolic markers had higher values in the groups that had normal ALT levels with FLD, especially high ALT levels with FLD, and abnormal ALT levels without FLD (Table 3 and Table S5). Higher exercise rates were observed in the normal group, relative to the groups with normal ALT levels with FLD, high ALT levels with FLD, or abnormal ALT levels without FLD (Table 3 and Table S5). Family history of diabetes was more common in the group with high ALT levels with FLD, relative to the normal group and the group with normal ALT levels with FLD (Table 3 and Table S5). Among alcoholic women, and relative to the normal group, many metabolic markers except the IFG onset rate had higher values in the groups that had normal ALT levels with FLD, high ALT with FLD, and abnormal ALT levels without FLD (Table 4 and Table S6). IFG onset rate had higher values in the group that had abnormal ALT levels without FLD than the normal group (Table S6). The rate of a snacking habit was higher in the normal group than in the other three groups (Table 4 and Table S6).
Risk of IFG onset in relation to FLD and ALT levels
The univariate analyses of men (Table 5) revealed that, relative to the normal group, there were significantly increased risks of IFG onset in the groups that had nonalcoholic normal ALT levels with FLD {hazard ratio (HR): 2.54, 95% CI: 1.93–3.33} and nonalcoholic high ALT levels with FLD (HR: 3.23, 95% CI: 2.54–4.08) (P‐trend < 0.01) (Table 5). Additionally, there was an increased risk in the group with high ALT levels without FLD (HR: 1.81, 95% CI: 1.29–2.54) (P < 0.01) (Table S7). Similarly, among the alcoholic groups of men, relative to the normal group, there were significantly increased risks of IFG onset in the groups that had normal ALT levels with FLD (HR: 3.14, 95% CI: 1.7–5.8) and high ALT levels with FLD (HR: 4.16, 95% CI: 2.39–7.22) (P‐trend < 0.01; Table 5). However, there was no significant association in the group with high ALT levels without FLD (Table S7).
Table 5.
Univariate and multivariate analyses of the onset of impaired fasting glucose among men
| Normal | Normal ALT levels and fatty liver disease | High ALT levels and fatty liver disease | P for trend | ||
|---|---|---|---|---|---|
| Nonalcoholic group | Incident rate (%) | 138/2,106 (6.6%) | 83/551 (15.1%) | 137/715 (19.2%) | |
| Crude HR (95% CI) | 1.00 | 2.54 (1.93–3.33) | 3.22 (2.54–4.08) | <0.01 | |
| Adjusted HR* (95% CI) | 1.00 | 1.54 (1.15–2.06) | 1.78 (1.33–2.38) | <0.01 | |
| Alcoholic group | Incident rate (%) | 11/71 (15.5%) | 14/68 (20.6%) | ||
| Crude HR (95% CI) | 1.00 | 3.14 (1.7–5.8) | 4.16 (2.39–7.22) | <0.01 | |
| Adjusted HR* (95% CI) | 1.00 | 0.92 (0.48–1.76) | 1.08 (0.55–2.09) | 0.93 |
Multivariate Cox proportional hazards regression analysis was adjusted for age (years), BMI (kg/m2), SBP (mmHg), FPG (mM), Cre (µM), UA (µM), TC (mM), TG (mM), exercise habits, snacking habits, and family history of diabetes. Differences were considered statistically significant for P < 0.05. HR, hazard ratio; CI, confidence interval; ALT, alanine aminotransferase; BMI, body mass index; SBP, systolic blood pressure; FPG, fasting plasma glucose; Cre, creatinine; UA, uric acid; TC, total cholesterol; TG, triglycerides.
Multivariate analyses of men were adjusted for age, BMI, SBP, FPG, TC, TG, UA, Cre, exercise and snacking habits, and family history of diabetes. The results revealed that, relative to the normal group, independently increased risks of IFG onset were observed in the groups that had nonalcoholic normal ALT levels with FLD {adjusted hazard ratio (aHR): 1.54, 95% CI: 1.15–2.06} and nonalcoholic high ALT levels with FLD (aHR: 1.78, 95% CI: 1.33–2.38) (P‐trend < 0.01) (Table 5). Additionally, there was an increased risk in the group with high ALT levels without FLD (aHR: 1.54, 95% CI: 1.07–2.23) (P < 0.01) (Table S7). However, among the alcoholic groups of men, none had elevated risks of IFG onset (Table 5 and Table S7).
In the normal group, nonalcoholic normal ALT levels with FLD group, and nonalcoholic high ALT levels with FLD group, the 1‐year IFG onset rates were 0.88%, 2.06%, and 1.87%; the 3‐year IFG onset rates were 3.2%, 7.16%, and 11.36%; the 5‐year IFG onset rates were 5.83%, 13.12%, and 17.76%; and the 10‐year IFG onset rates were 10.68%, 29.12%, and 30.34%, respectively. In the nonalcoholic abnormal ALT levels without FLD group, the 1‐year IFG onset rate was 0.5%, the 3‐year IFG onset rate was 5.7% the 5‐year IFG onset rate was 8.4%, and the 10‐year IFG onset rate was 21.98%.
Moreover, in men, the ALT level at baseline was correlated with the ALT level at endpoint in participants with the onset of IFG (r = 0.633, P < 0.01).
Univariate analyses of women (Table 6) revealed that, relative to the normal group, there were significantly increased risks of IFG onset in the groups that had NAFLD {normal ALT levels with FLD (HR: 4.6, 95% CI: 2.96–7.17) and nonalcoholic high ALT levels with FLD (HR: 8.26, 95% CI: 5.54–12.29) (P‐trend < 0.01)}, and alcoholic high ALT levels with FLD (HR: 9.82, 95% CI: 1.36–71.19). However, among women, only the nonalcoholic normal ALT levels with FLD group had independently elevated risks of IFG onset (aHR: 1.67, 95% CI: 1.03–2.7) (Table 6 and Table S8).
Table 6.
Univariate and multivariate analyses of the onset of impaired fasting glucose among women
| Normal | Normal ALT levels and fatty liver disease | High ALT levels and fatty liver disease | P for trend | ||
|---|---|---|---|---|---|
| Nonalcoholic group | Incident rate (%) | 80/3,588 (2.2%) | 26/280 (9.3%) | 35/235 (14.9%) | |
| Crude HR (95% CI) | 1.00 | 4.6 (2.96–7.17) | 8.26 (5.54–12.29) | <0.01 | |
| Adjusted HR* (95% CI) | 1.00 | 1.67 (1.03–2.7) | 1.03 (0.6–1.77) | 0.08 | |
| Alcoholic group | Incident rate (%) | 1/15 (6.7%) | 1/11 (9.1%) | ||
| Crude HR (95% CI) | 1.00 | 4.11 (0.57–29.61) | 9.82 (1.36–71.19) | 0.03 | |
| Adjusted HR (95% CI) | 1.00 | 0.93 (0.11–7.9) | 1.32 (0.15–11.61) | 0.96 |
Multivariate Cox proportional hazards regression analysis was adjusted for age (years), BMI (kg/m2), SBP (mmHg), FPG (mM), Cre (µM), UA (µM), TC (mM), TG (mM), exercise habits, snacking habits, and family history of diabetes. Differences were considered statistically significant for P < 0.05. HR, hazard ratio; CI, confidence interval; ALT, alanine aminotransferase; BMI, body mass index; SBP, systolic blood pressure; FPG, fasting plasma glucose; Cre, creatinine; UA, uric acid; TC, total cholesterol; TG, triglycerides.
In the normal group and the nonalcoholic normal ALT levels with FLD group, the 1‐year IFG onset rates were 0.14% and 1.11%; the 3‐year IFG onset rates were 0.56% and 3.65%; the 5‐year IFG onset rates were 1.29% and 8.27%; and the 10‐year IFG onset rates were 3.88% and 15.03%, respectively.
In women, the ALT level at baseline was correlated with the ALT level at endpoint in participants with the onset of IFG (r = 0.635, P < 0.01).
DISCUSSION
The present study revealed that an elevated risk of IFG onset was associated with nonalcoholic men who had normal or high ALT levels with FLD and had high ALT levels without FLD, and nonalcoholic women who had normal ALT levels with FLD, independent of any potential confounding factors. However, among alcoholic men and women, as well as among nonalcoholic women who had high ALT levels with and without FLD, none of the subgroups exhibited an elevated risk of IFG onset. Additionally, in men and women, the ALT level at baseline was correlated with the ALT level at endpoint in participants with the onset of IFG. These results suggest that, especially in men, FLD in nonalcoholic patients is a risk factor for developing impaired glucose tolerance, even in individuals with normal ALT levels.
Several studies have examined the relationship between the onset of glucose intolerance and FLD. Mantovani et al. performed a meta‐analysis of the association between NAFLD and the onset of diabetes based on data from 19 observational studies with 296,439 participants and a median follow‐up of 5 years 10 . The results revealed that relative to participants without NAFLD, NAFLD was associated with the onset of diabetes, and the more severe NAFLD that was identified using ultrasonography or a NAFLD fibrosis score, was related to a greater risk of developing incident diabetes 10 . However, it remains unclear whether NAFLD patients with normal ALT levels have an increased risk of developing glucose intolerance.
The association between NAFLD and the development of IFG may be related to hepatokines, such as fetuin A, fetuin B, retinol‐binding protein 4, and selenoprotein P, which are secreted from hepatocytes and upregulated in the state of hepatic steatosis. These proteins also induce insulin resistance by suppressing the insulin‐induced tyrosine phosphorylation of the insulin receptor or inactivating adenosine monophosphate‐activated protein kinase 26 . FLD is also associated with increases in diacylglycerols and ceramides, which are mediators of lipid‐induced hepatic insulin resistance 27 . In this context, diacylglycerols activate the protein kinase C isoforms and activation of the ε isoform leads to the phosphorylation of the insulin receptor at Thr1160, which inhibits its tyrosine kinase activity. Moreover, impaired hepatocellular insulin signaling decreases glycogen synthesis and de novo lipogenesis, while increasing gluconeogenesis. Ceramide‐induced protein kinase C‐ζ activation also exacerbates the translocation of AKT to the plasma membrane and prevents AKT from participating in insulin signaling. Additionally, ceramide activates protein phosphatase 2A and dephosphorylates AKT resulting in the inactivation of AKT.
Interestingly, our results indicate that alcoholic fatty liver is not a risk factor for the development of IFG, which is supported by findings reported in previous studies. Fueki et al. evaluated 1,029 men who had undergone medical check‐ups and reported that moderate alcohol intake improved insulin resistance in healthy men, regardless of obesity 11 . Akahane et al. performed a cross‐sectional study of 2,461 men who had undergone regular health check‐ups and also showed that chronic alcohol intake was inversely related to insulin resistance among Japanese men, regardless of the type of alcoholic beverage consumed 12 . Furthermore, chronic alcohol intake increases the hepatic levels of glutathione, which is known to increase the production of the hepatic insulin sensitizing substance and improve the sensitivity to insulin 28 , 29 . Nevertheless, other studies have indicated that high alcohol intake increased the risk of type 2 diabetes 13 , 14 and impaired β‐cell function 30 , 31 . Therefore, further studies are needed to clarify these potential relationships.
The differences we observed between men and women might be associated with sex hormones and the distribution of adipose tissue 32 , 33 , 34 . In this context, estrogen maintains glucose homeostasis by reducing hepatic glucose production and protecting the pancreatic β‐cell function/survival and insulin secretion. Furthermore, estrogen affects adipose tissue biology and is associated with the prevention of obesity and adipose tissue distribution 32 , as men tend to accumulate visceral adipose tissue and women tend to accumulate peripheral and subcutaneous adipose tissue. Moreover, increased visceral adipose tissue exacerbates insulin sensitivity in the muscle and liver by decreasing adiponectin production and increasing proinflammatory cytokine production 34 , 35 , 36 , 37 . The present study included only a small number of women, which may explain why nonalcoholic fatty liver and abnormal ALT levels were not associated with the development of IFG.
The strength of our study lies in the fact that it was based on the general Japanese population and that data were nearly complete (only five data points were missing). However, there were also several limitations to consider. First, we identified only 262 alcoholic individuals (203 men and 59 women), which may be the reason for the lack of significant associations between IFG onset and FLD or high ALT levels. Second, abdominal ultrasonography is a useful diagnostic modality with fairly high sensitivity and specificity for fatty liver 38 , 39 . However, it has low sensitivity to detect fatty liver with advanced liver fibrosis such as burn‐out NASH, and the actual percentage of FLD may be underestimated 40 , 41 . Additionally, we did not have access to data regarding FLD severity, which precluded an analysis of the association between FLD severity and the onset of IFG. Third, self‐reported data were used for several of the investigated factors, which may decrease the accuracy of our findings. Fourth, we could not examine the relationship between FLD and the development of diabetes mellitus because the total number of patients with diabetes onset was 74, which was not sufficient to divide them into seven groups by sex and examine the aforementioned relationship. Fifth, we only collected data from annual health check‐ups, which means that the data were not truly continuous. Finally, we only considered Japanese individuals, and studies in other populations are necessary to confirm the generalizability of our results.
In conclusion, the present study revealed that FLD in nonalcoholic patients increased the risk of developing glucose intolerance, even if the ALT level was normal. Therefore, regardless of their ALT levels, nonalcoholic patients with FLD require care to prevent the onset of prediabetes and reduce the risk of the development of diabetes and cardiovascular disease.
DISCLOSURE
The author declares no conflict of interest.
Supporting information
Table S1 | Categories based on average alcohol consumptions (Men).
Table S2 | Categories based on average alcohol consumptions (Women).
Table S3 | Baseline characteristics of nonalcoholic men with high ALT and non‐fatty liver disease.
Table S4 | Baseline characteristics of alcoholic men with high ALT and non‐fatty liver disease.
Table S5 | Baseline characteristics of nonalcoholic women with high ALT and non‐fatty liver disease.
Table S6 | Baseline characteristics of alcoholic women with high ALT and non‐fatty liver disease.
Table S7 | Univariate and multivariate analyses of the onset of impaired fasting glucose among men.
Table S8 | Univariate and multivariate analyses of the onset of impaired fasting glucose among women.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number JP19K11743 and a research grant from Ehime University. We would like to thank Editage (www.editage.com) for English language editing.
J Diabetes Investig. 2021; 12: 1890–1898
REFERENCES
- 1. Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047–1053. [DOI] [PubMed] [Google Scholar]
- 2. Partanen J, Niskanen L, Lehtinen J, et al. Natural history of peripheral neuropathy in patients with non‐insulin‐dependent diabetes mellitus. N Engl J Med 1995; 333: 89–94. [DOI] [PubMed] [Google Scholar]
- 3. Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes 2015; 6: 1246–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shima T, Uto H, Ueki K, et al. Hepatocellular carcinoma as a leading cause of cancer‐related deaths in Japanese type 2 diabetes mellitus patients. J Gastroenterol 2019; 54: 64–77. [DOI] [PubMed] [Google Scholar]
- 5. Kim CH, Park JY, Lee KU, et al. Fatty liver is an independent risk factor for the development of type 2 diabetes in Korean adults. Diabet Med 2008; 25: 476–481. [DOI] [PubMed] [Google Scholar]
- 6. Yamada T, Fukatsu M, Suzuki S, et al. Fatty liver predicts impaired fasting glucose and type 2 diabetes mellitus in Japanese undergoing a health checkup. J Gastroenterol Hepatol 2010; 25: 352–356. [DOI] [PubMed] [Google Scholar]
- 7. Miyake T, Hirooka M, Yoshida O, et al. Differences in the risk of fatty liver for onset of impaired fasting glucose according to baseline plasma glucose levels. J Gastroenterol 2017; 52: 237–244. [DOI] [PubMed] [Google Scholar]
- 8. Shibata M, Kihara Y, Taguchi M, et al. Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle‐aged Japanese men. Diabetes Care 2007; 30: 2940–2944. [DOI] [PubMed] [Google Scholar]
- 9. Li CH, Chou YT, Shen WC, et al. Increased risks of different grades of non‐alcoholic fatty liver disease in prediabetic subjects with impaired fasting glucose and glucose tolerance, including the isolated glycosylated hemoglobin levels of 5.7–6.4% in a Chinese population. J Diabetes Investig 2020; 11: 1336–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mantovani A, Byrne CD, Bonora E, et al. Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: a meta‐analysis. Diabetes Care 2018; 41: 372–382. [DOI] [PubMed] [Google Scholar]
- 11. Fueki Y, Miida T, Wardaningsih E, et al. Regular alcohol consumption improves insulin resistance in healthy Japanese men independent of obesity. Clin Chim Acta 2007; 382: 71–76. [DOI] [PubMed] [Google Scholar]
- 12. Akahane T, Namisaki T, Kaji K, et al. Chronic alcohol consumption is inversely associated with insulin resistance and fatty liver in Japanese males. Nutrients 2020; 12: 1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carlsson S, Hammar N, Grill V, et al. Alcohol consumption and the incidence of type 2 diabetes: a 20‐year follow‐up of the Finnish twin cohort study. Diabetes Care 2003; 26: 2785–2790. [DOI] [PubMed] [Google Scholar]
- 14. Waki K, Noda M, Sasaki S, et al. Alcohol consumption and other risk factors for self‐reported diabetes among middle‐aged Japanese: a population‐based prospective study in the JPHC study cohort. Diabet Med 2005; 22: 323–331. [DOI] [PubMed] [Google Scholar]
- 15. Okamoto M, Miyake T, Kitai K, et al. Cigarette smoking is a risk factor for the onset of fatty liver disease in nondrinkers: a longitudinal cohort study. PLoS One 2018; 13: e0195147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kojima S, Watanabe N, Numata M, et al. Increase in the prevalence of fatty liver in Japan over the past 12 years: analysis of clinical background. J Gastroenterol 2003; 38: 954–961. [DOI] [PubMed] [Google Scholar]
- 17. Wai JW, Fu C, Wong VW. Confounding factors of non‐invasive tests for nonalcoholic fatty liver disease. J Gastroenterol 2020; 55: 731–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol 2017; 112: 18–35. [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization . Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. World Health Organization, Geneva, 2006. [Google Scholar]
- 20. Kuzuya T, Nakagawa S, Satoh J, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Res Clin Pract 2002; 55: 65–85. [DOI] [PubMed] [Google Scholar]
- 21. American Diabetes Association . Screening for type 2 diabetes. Diabetes Care 2003; 26: S21–S24. [DOI] [PubMed] [Google Scholar]
- 22. Harita N, Hayashi T, Sato KK, et al. Lower serum creatinine is a new risk factor of type 2 diabetes: the Kansai healthcare study. Diabetes Care 2009; 32: 424–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sakurai M, Nakamura K, Miura K, et al. Family history of diabetes, lifestyle factors, and the 7‐year incident risk of type 2 diabetes mellitus in middle‐aged Japanese men and women. J Diabetes Investig 2013; 4: 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Waki K, Noda M, Sasaki S, et al. Alcohol consumption and other risk factors for self‐reported diabetes among middle‐aged Japanese: a population‐based prospective study in the JPHC study cohort I. Diabet Med 2005; 22: 323–331. [DOI] [PubMed] [Google Scholar]
- 25. Miyake T, Kumagi T, Furukawa S, et al. Hyperuricemia is a risk factor for the onset of impaired fasting glucose in men with a high plasma glucose level: a community‐based study. PLoS One 2014; 9: e107882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meex RCR, Watt MJ. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat Rev Endocrinol 2017; 13: 509–520. [DOI] [PubMed] [Google Scholar]
- 27. Petersen MC, Shulman GI. Roles of diacylglycerols and ceramides in hepatic insulin resistance. Trends Pharmacol Sci 2017; 38: 649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang CM, Carlson GP. Effects of ethanol on glutathione conjugation in rat liver and lung. Biochem Pharmacol 1991; 41: 923–929. [DOI] [PubMed] [Google Scholar]
- 29. Guarino MP, Afonso RA, Raimundo N, et al. Hepatic glutathione and nitric oxide are critical for hepatic insulin‐sensitizing substance action. Am J Physiol Gastrointest Liver Physiol 2003; 284: G588–G594. [DOI] [PubMed] [Google Scholar]
- 30. Yoo MG, Kim HJ, Jang HB, et al. The association between alcohol consumption and β‐Cell function and insulin sensitivity in Korean population. Int J Environ Res Public Health 2016; 13: 1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yue F, Zhang X, Zhang H, et al. Association of alcohol consumption with the impaired β‐cell function independent of body mass index among Chinese men. Endocr J 2012; 59: 425–433. [DOI] [PubMed] [Google Scholar]
- 32. Louet JF, LeMay C, Mauvais‐Jarvis F. Antidiabetic actions of estrogen: insight from human and genetic mouse models. Curr Atheroscler Rep 2004; 6: 180–185. [DOI] [PubMed] [Google Scholar]
- 33. Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med 2009; 6: 60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamauchi T, Kamon J, Waki H, et al. The fat‐derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001; 7: 941–946. [DOI] [PubMed] [Google Scholar]
- 35. Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 2003; 46: 459–469. [DOI] [PubMed] [Google Scholar]
- 36. Chawla A, Nguyen KD, Goh YPS. Macrophage‐mediated inflammation in metabolic disease. Nat Rev Immunol 2011; 11: 738–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hui E, Xu A, Bo Yang H, et al. Obesity as the common soil of non‐alcoholic fatty liver disease and diabetes: role of adipokines. J Diabetes Investig 2013; 4: 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Joy D, Thava VR, Scott BB. Diagnosis of fatty liver disease: is biopsy necessary? Eur J Gatroenterol Hepatol 2003; 15: 539–543. [DOI] [PubMed] [Google Scholar]
- 39. Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology 2007; 46: 582–589. [DOI] [PubMed] [Google Scholar]
- 40. Perez NE, Siddiqui FA, Mutchnick MG, et al. Ultrasound diagnosis of fatty liver in patients with chronic liver disease. A retrospective observational study. J Clin Gastroenterol 2007; 41: 624–629. [DOI] [PubMed] [Google Scholar]
- 41. Tobari M, Hashimoto E, Yatsuji S, et al. Imaging of nonalcoholic steatohepatitis: advantages and pitfalls of ultrasonography and computed tomography. Inter Med 2009; 48: 739–746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Categories based on average alcohol consumptions (Men).
Table S2 | Categories based on average alcohol consumptions (Women).
Table S3 | Baseline characteristics of nonalcoholic men with high ALT and non‐fatty liver disease.
Table S4 | Baseline characteristics of alcoholic men with high ALT and non‐fatty liver disease.
Table S5 | Baseline characteristics of nonalcoholic women with high ALT and non‐fatty liver disease.
Table S6 | Baseline characteristics of alcoholic women with high ALT and non‐fatty liver disease.
Table S7 | Univariate and multivariate analyses of the onset of impaired fasting glucose among men.
Table S8 | Univariate and multivariate analyses of the onset of impaired fasting glucose among women.


