Fig. 2.
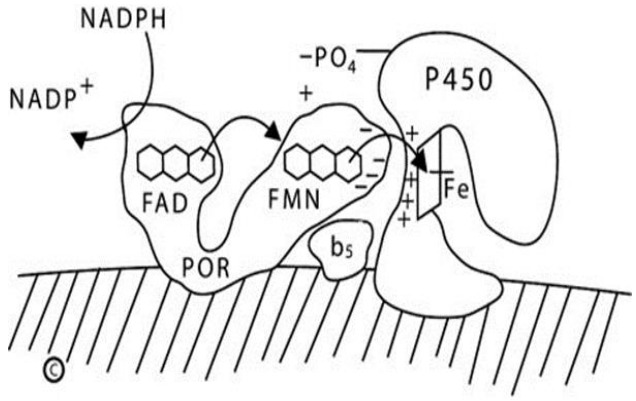
Diagram of type 2 (microsomal) P450 enzymes. The endoplasmic reticulum membrane is indicated by the hatched area; both P450 oxidoreductase (POR) and the P450 are membrane-bound. NADPH interacts with the flavin adenine dinucleotide (FAD) domain of POR and donates a pair of electrons to the FAD moiety. Electron receipt elicits a conformational change, permitting the isoalloxazine rings of the FAD and flavin mononucleotide (FMN) moieties to come close together, permitting the electrons to transfer from the FAD to the FMN. Electron receipt by the FMN reverts the POR protein to its original, open conformation, permitting the FMN domain to interact with the redox partner binding site of the P450 by electrostatic charge interactions. The electrons reach the iron atom of the heme group of the P450, permitting catalysis. For some reactions catalyzed by some P450 enzymes, notably the 17,20-lyase activity of human P450c17, cytochrome b5 acts allosterically to promote increased activity. NADP+, nicotinamide adenine dinucleotide phosphate; NADPH, reduced adenine dinucleotide phosphate.
