On behalf of the American Sexually Transmitted Diseases Association, we discuss benefits and challenges of direct-to-consumer test services for sexually transmitted infections and offer recommendations for future directions.
Abstract
Direct-to-consumer test services have gained popularity for sexually transmitted infections in recent years, with substantially increased use as a result of the SARS-CoV-2 (CoVID-19) global pandemic. This method of access has been variously known as “self-testing,” “home testing,” and “direct access testing.” Although these online services may be offered through different mechanisms, here we focus on those that are consumer-driven and require self-collected samples, and sample shipment to a centralized laboratory without involvement of health care providers and/or local health departments. We provide the American Sexually Transmitted Diseases Association's position on utilization of these services and recommendations for both consumers and health care providers.
BACKGROUND
Direct-to-consumer (DTC) test services for sexually transmitted infections (STIs) are available in many configurations. This position statement focuses on online test services with DTC purchasing options that involve self-collection of samples in a nonclinical setting and transport (through mail or direct drop off) of samples to a testing laboratory. These tests have been variously referred to as “online testing,” “self-testing,” “home testing,” and “direct access testing.” Here we will use the phrase DTC testing. Direct-to-consumer products include access to testing kits directly through a retail outlet or through Web sites offering STI screening services. From online vendors, collection materials are shipped to consumers who then collect a sample to be sent to a laboratory for testing. Transport to the laboratory may be accomplished via drop-off at a laboratory or health care facility or, more commonly, via shipment or courier service directly to the testing laboratory. We understand that similar testing access is available through alternate mechanisms such as telehealth or via local health departments in some jurisdictions, but these are not the focus of this position statement because these are directed by a health care entity who has a working relationship with the laboratory and established mechanisms for handling results and providing care. Here we focus on the advantages and challenges, along with our recommendations relating to DTC STI testing.
Advantages of DTC Test Services
Modalities of DTC testing can potentially address documented barriers to STI testing including inconvenience, privacy concerns, and the sense of stigma associated with seeking STI-specific care. Compared with face-to-face care, DTC testing services are perceived as offering more privacy, a primary concern among teens and young adults.1–3 They also address concerns of embarrassment or discomfort around a discussion about sexual history and/or sexual health examination, both of which are cited as major barriers to care utilization.4 The fear of stigma, a well-documented concern around STI testing and management,3,5 may be alleviated by the use of DTC test services. Furthermore, the use of DTC test services could improve access to testing for persons that otherwise would not attend clinics that provide STI services because of limited access.4–7
Direct-to-consumer test services may also be advantageous to clinicians and clinical programs. The Centers for Disease Control and Prevention8 and the US Preventive Services Task Force9 recommend annual STI screening for sexually active women younger than 25 years and more frequent screening for persons on preexposure prophylaxis.10 Direct-to-consumer test services may reduce the burden on health care providers who cite insufficient time and staff as barriers to performing routine STI screening, particularly among asymptomatic persons.11,12 The COVID-19 pandemic represents a situation in which face-to-face services were severely limited, and many clinics have relied on online mechanisms for providing STI testing remotely. This may result in an increased demand for access to testing services through commercial entities due to increased comfort with the process.
Importantly, DTC STI test services have the potential to provide substantial support to the overall public health effort to control STIs. Identifying STIs, particularly asymptomatic cases, is a critical component of reducing transmission13; thus, any program that increases rates of testing may enhance overall control efforts. Several studies have suggested that self-sampling may increase uptake of chlamydia and gonorrhea testing,6,14,15 and it stands to reason that consumers may also embrace DTC testing. It is conceivable that this strategy could improve the quality of partner management services by directing contacts to use DTC services. This could facilitate access to more convenient and private testing, while also reducing the burden on public health agencies who currently manage partners in clinical settings.
Challenges of DTC
Although the potential for expanding the reach of STI screening by utilization of DTC services is great, there are challenges that need to be addressed in order to ensure the quality of testing, equity of access, communication of results, consumer and partner management, and comprehensive surveillance reporting. Data suggest that there is room for improvement in the process of managing persons with positive results identified via DTC testing16 (Cannon et al., this issue).
Accessibility may improve because of DTC; however, adolescents and young adults living with others cite privacy concerns related to mailed collection kits.17–19 In addition, although DTC STI test services may increase access for some populations, other populations, for example, persons experiencing housing insecurity, may not be able overcome potentially high costs and requirements for a mailing address and Internet access.20 Even for those living in stable housing, about 8% of Americans lack Internet access at home.21 This is particularly concerning given that these same populations are disproportionately impacted by STIs.8 Finally, the cost of DTC online testing services varies widely, which also impacts accessibility. Chlamydia and gonorrhea testing prices range from $49 to $189 or higher. Bundled testing that includes other STIs can cost >$400, which may be prohibitive to the persons most in need of increased access to care, including those who already cite cost as a barrier to STI testing.2,22 Furthermore, DTC services may not be reimbursable under some insurance plans because the testing is not ordered by a health care provider.
A second challenge associated with DTC STI testing is ensuring that appropriate testing is being ordered and performed. Direct-to-consumer online testing sites offer an array of testing options and may use algorithms or artificial intelligence, rather than health care providers, to assist the consumer with selecting the most appropriate test or test bundle. However, recommendations from the DTC service to the consumer frequently lack the clarity and details needed for an informed decision about which test/s is/are most appropriate and the follow-up that may be required16 (Cannon et al., this issue). Bundled testing often includes unnecessary tests or tests that are only in recommended in very specific circumstances. For example, bundles may include molecular testing for Ureaplasma species or Mycoplasma hominis for which there are no screening or treatment recommendations, or Mycoplasma genitalium for which testing is only recommended in men with persistent urethritis or women with cervicitis or pelvic inflammatory disease.23 These bundles often include options for herpes simplex virus molecular testing and/or testing for Treponema pallidum from urine or unobserved self-collected vaginal swabs, although such testing should only be performed from specimens obtained directly from lesions.13,24 Finally, consumer knowledge of necessary tests may be limited, which could result in overtesting out of an abundance of caution or undertesting due to a lack of understanding of their risk for certain infections.
An important and understudied issue related to DTC testing is a potential lack of diagnostic reliability and validity. Reliability of the results, such as receiving incorrect or inconclusive results19,25,26 and concerns about collecting specimens appropriately,19,26,27 has often been reported with DTC services. Other potential service issues that have not been fully documented include whether testing materials/kits arrive to the correct location once purchased; the quality of the shipped samples (which may be affected by transport conditions); and whether results are ever received and, if received, the time from sample collection to reporting of test results. Currently, the testing quality and diagnostic reliability remain unclear, and no commercially available assays have a Food and Drug Administration (FDA)–approved/cleared claim for unobserved self-collected specimens in a nonclinical setting. The methodology used by the testing laboratory to determine the validity of their collection, transport, and testing processes is often not made public, and obtaining details about internal laboratory validation may be difficult for both consumers and providers. The sensitivity and specificity of the assay used are often not available on service Web sites, and when present, it may be difficult for consumers to interpret, particularly in the absence of understanding the entire validation approach. This creates uncertainty about performance characteristics for the assay used, which hampers appropriate clinical management of the consumer.
Reporting and taking action on test results is imperative. Results must be reported to the consumer in clear language and must include information about the consumers' next steps. Nearly 36% of Americans suffer from low health literacy28 and may be more likely to trust social media and blog posts for health information,29 which poses a challenge for delivering DTC test results. Results of testing for 3 common STIs, chlamydia, gonorrhea, and trichomoniasis, are relatively simple to interpret with positive results requiring treatment and negative results requiring no further evaluation. However, results for other STIs necessitate interpretation that is often dependent on data from the clinical and medical history. For example, interpreting syphilis serologies requires knowledge of sexual history, previous exposures/diagnoses, information about the diagnostic test used, and/or confirmatory test results.13 In cases where confirmatory tests are not performed, test results may not be interpretable. For appropriate clinical management, especially in cases where the recommended treatment may involve injectable medications, direct interaction with a health care provider may be required.
Finally, communication of these reportable conditions by commercial testing services to public health providers and agencies may be problematic and can result in delayed time to treatment, and missed opportunities for partner services, surveillance, and prevention of community transmission.16 Linkage to care when using these services is difficult to ascertain, and persons may not receive recommended treatment. In addition, the process for health care providers to manage results from online test services remains unclear potentially resulting in redundant or unnecessary confirmatory testing. This redundant testing to confirm test results may increase cost and time to treatment, thereby reducing the potential benefits from DTC testing. Finally, if reporting to public health agencies is delayed or not done, there may be gaps in partner services and treatment, impacting key recommendations for providing quality STI clinical services.30
RECOMMENDATIONS
The American Sexually Transmitted Diseases Association (ASTDA) supports leveraging appropriate tools to reduce the burden of STIs in all populations. Recommendations in Figure 1 address 5 important areas to reduce the potential for harm and improve outcomes for consumers, clinicians, and public health practitioners: consumer protection and empowerment, test selection issues, laboratory-specific issues, clinical management concerns, and policy recommendations. These recommendations are directed to our STI policy, research, practice, laboratory, and clinical partners. We hope that consumers will request, and commercial services will engage in this process of increased transparency to ensure access to high-quality and effective STI testing.
Figure 1.
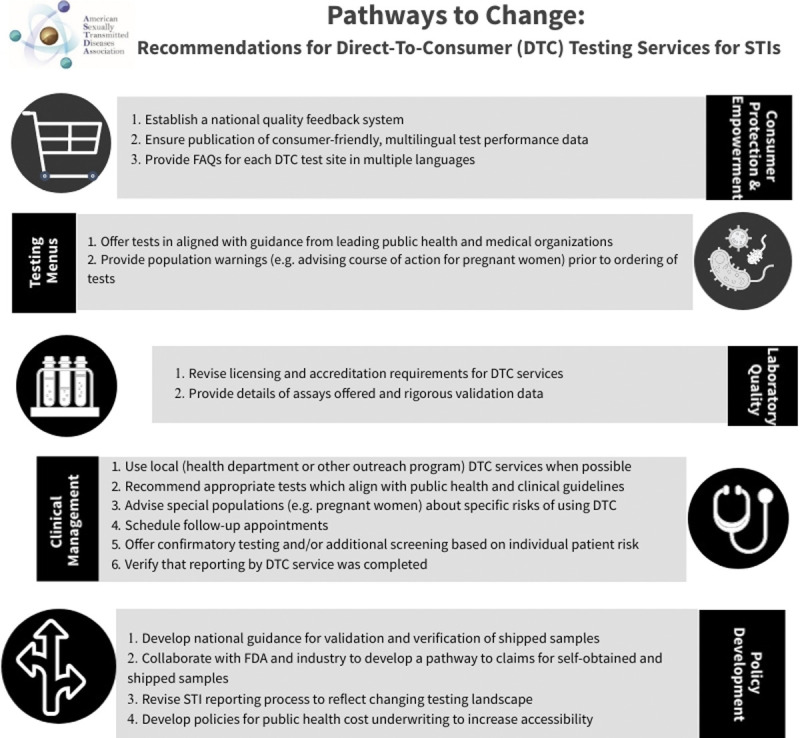
The pathway to change summarizes the recommendations for DTC STI testing.
Consumer Protection and Empowerment Recommendations
These recommendations are focused on achieving information transparency and quality. Specific recommendations are as follows:
-
o
Establish a national feedback system to publicly identify Web sites and service providers, their quality, and customer experience
-
o
DTC service providers should
-
o
publish consumer-friendly, multilingual validation data and their interpretation, as well as detailed information about sample collection, testing processes, and potential for insurance reimbursement, and
-
o
publish and maintain up-to-date frequently asked questions (FAQs) on each test service Web site. FAQs should also be available to download. The key is to provide important information for health care providers and public health practitioners who make management decisions based on these test results. Information for these FAQs should be developed in collaboration with all stakeholders, and may include
-
▪
expected time to results,
-
▪
evidence of involvement of a knowledgeable health care provider (MD/NP/PA/DO) in test selection and treatment options,
-
▪
the performance characteristics of each test and how these characteristics were determined,
-
▪
description of the mechanism for managing positive test results,
-
▪
linkage to STI health care specialists to answer consumer questions,
-
▪
insurance reimbursement and consumer financial liability, and
-
▪
prevention services information and referrals.
-
▪
-
o
Test Selection Recommendations
Direct-to-consumer testing services and laboratory partners should only offer tests that are aligned with public health agencies (e.g., Centers for Disease Control and Prevention and US Preventive Services Task Force) and professional societies' (e.g., the American College of Obstetrics and Gynecology and American Academy of Pediatrics) guidance. Recommendations are as follows:
-
o
Organisms for which we have no screening or diagnostic recommendations (e.g., Ureaplasma species and M. hominis) should not be tested for via online services. They should not be offered as part of an STI screening panel for diagnostic/clinical management.
-
o
Screening among asymptomatic populations should be limited to those infections for which there is demonstrated value to screening (e.g., asymptomatic screening for M. genitalium is not currently recommended).
-
o
Population-specific warnings should be clearly communicated at the time a test is ordered (e.g., pregnant women should be encouraged to see an OB/GYN or their primary care provider for testing).
Laboratory Recommendations
These recommendations focus on quality of laboratory testing that experts in the field must do the following:
-
o
Work with Centers for Medicare & Medicaid Services, the agency that oversees Clinical Laboratory Improvements Amendment regulations to tighten regulatory requirements for DTC services that involve self-collection and transport of specimens
-
o
Work with College of American Pathologists and other programs to strengthen the accreditation processes by requiring rigorous validation of assay reliability and quality of services
-
o
Validation data should be available for review by any consumers: health care providers or persons using the DTC services.
-
o
Recommendations for Clinical Management
These recommendations are to help assist with decision making when interpreting test utility and results as well as verifying functionality of local reporting processes.
-
o
General recommendations for providers
-
o
Become familiar with the available high-quality DTC services should patients request specific recommendations
-
o
Suggest appropriate tests as recommended by public health or professional guidelines
-
o
Advise in-person testing for patients with high-risk of potential negative outcomes (e.g., pregnant women) related to missed infections
-
o
-
o
Recommendations for providers related to interpretation of results:
-
o
Provide follow-up for interpretation of results (telehealth/in-person).
-
o
Be cautious when interpreting results unless access to test performance data is available.
-
o
Provide additional screening/testing when necessary (i.e., DTC chlamydia testing without gonorrhea testing) and provide appropriate risk reduction counseling such as HIV preexposure prophylaxis.
-
o
Offer confirmatory testing where appropriate (e.g., for HIV and syphilis serology results) but do not let confirmatory testing be a barrier to treatment.
-
o
Inquire about the use of DTC services during collection of routine medical history, which may help illuminate patient medical concerns not otherwise expressed.
-
o
Ascertain the treatment prescribed by the DTC to ensure appropriate therapy was provided for any identified pathogens.
-
o
Verify reporting of notifiable infections identified by DTC testing. If reporting is not being done, work with local and state public health agencies to close the gap.
-
o
Policy Recommendations
Recommendations are made to help advance our policy and practice to use DTC testing to improve access to quality services that will assure health value. They are as follows:
-
o
Develop national guidance for validation and/or verification of self-obtained and shipped samples for STI testing. This guidance can be developed in partnership with the Association of Public Health Laboratories, ASTDA, and others.
-
o
Design novel STI reporting processes to better support DTC testing services reporting.
-
o
Work with the FDA and industry to try to develop a path toward achieving claims for self-obtained and shipped samples.
-
o
Develop policies that provide public health underwriting of the cost of testing or increase the utilization of public health laboratories as a mechanism for reducing health inequities and improving access.
THE WAY FORWARD
Sexually transmitted infection professionals have a collective opportunity to expand the reach of STI control efforts by using high-quality DTC testing services. The ASTDA supports this development and recommends actions to assure consumer protection and empowerment. This opportunity not only has the potential to improve the quality and impact of DTC STI testing services, but will also be relevant to over-the-counter STI tests when such tests become cleared for use by the FDA. Optimizing the process for DTC test services will prepare us for diagnostic testing modalities currently in use, in the pipeline, or yet to be envisioned. Doing so in a way that involves all stakeholders (e.g., consumers, public health practitioners, health care providers, laboratories, testing services providers, and researchers) will serve to strengthen the future of STI testing services for all.
Footnotes
Sources of Funding: No funding support was received for this article.
Conflict of Interest: C.E., B.M., and S.B.G. have nothing to disclose. C.N.P. reports receiving honorarium/consulting fees paid directly to her by Becton Dickinson. A.M.G. reports receiving funding to her institution from Abbott, BD, Cepheid, DiaSorin, IDEXX, Illumina, Hologic, Luminex, National Jewish Health, Roche, SpeeDx, ThermoFisher Scientific, and Qiagen. B.V.D.P. reports receiving funding to her institution from Abbott, Becton Dickinson, binx health, Cepheid, Hologic, Rheonix, Roche, and SpeeDx, and honorarium/consulting fees paid directly to her from Abbott, BD, and Roche.
Contributor Information
Cara Exten, Email: cara.exten@psu.edu.
Casey N. Pinto, Email: cpinto@psu.edu.
Anne M. Gaynor, Email: anne.gaynor@aphl.org.
Beth Meyerson, Email: bmeyerson@arizona.edu.
Stacey B. Griner, Email: stacey.griner@unthsc.edu.
REFERENCES
- 1.Ford CA, Best D, Miller WC. The pediatric forum: Confidentiality and adolescents' willingness to consent to sexually transmitted disease testing. Arch Pediatr Adolesc Med 2001; 155:1072–1073. [PubMed] [Google Scholar]
- 2.Cuffe KM Newton-Levinson A Gift TL, et al. Sexually transmitted infection testing among adolescents and young adults in the United States. J Adolesc Health 2016; 58:512–519. [DOI] [PubMed] [Google Scholar]
- 3.Fleming C Drennan VM Kerry-Barnard S, et al. Understanding the acceptability, barriers and facilitators for chlamydia and gonorrhoea screening in technical colleges: Qualitative process evaluation of the “Test n Treat” trial. BMC Public Health 2020; 20:1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert M Thomson K Salway T, et al. Differences in experiences of barriers to STI testing between clients of the Internet-based diagnostic testing service GetCheckedOnline.com and an STI clinic in Vancouver, Canada. Sex Transm Infect 2019; 95:151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tilson EC Sanchez V Ford CL, et al. Barriers to asymptomatic screening and other STD services for adolescents and young adults: focus group discussions. BMC Public Health 2004; 4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kersaudy-Rahib D Lydié N Leroy C, et al. Chlamyweb Study II: A randomised controlled trial (RCT) of an online offer of home-based Chlamydia trachomatis sampling in France. Sex Transm Infect 2017; 93:188–195. [DOI] [PubMed] [Google Scholar]
- 7.Meyerson BE Davis A Reno H, et al. Existence, distribution, and characteristics of STD clinics in the United States, 2017. Public Health Rep 2019; 134:371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention . Sexually Transmitted Disease Surveillance, 2018. Atlanta, GA: US Department of Health and Human Services, 2019. [Google Scholar]
- 9.United States Preventive Services Task Force . Final Update Summary: Chlamydia and Gonorrhea Screening. 2016.
- 10.Centers for Disease Control and Prevention . Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 update: A clinical practice guideline. US Public Health Service 2018. [Google Scholar]
- 11.Mark H Irwin K Sternberg M, et al. Providers' perceived barriers to sexually transmitted disease care in 2 large health maintenance organizations. Sex Transm Dis 2008; 35:184–189. [DOI] [PubMed] [Google Scholar]
- 12.Carter JW Jr. Hart-Cooper GD Butler MO, et al. Provider barriers prevent recommended sexually transmitted disease screening of HIV-infected men who have sex with men. Sex Transm Dis 2014; 41:137–142. [DOI] [PubMed] [Google Scholar]
- 13.Workowski KA Bolan GA, Centers for Disease Control and Prevention . Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 14.Söderqvist J Gullsby K Stark L, et al. Internet-based self-sampling for Chlamydia trachomatis testing: A national evaluation in Sweden. Sex Transm Infect 2020; 96:160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook RL Østergaard L Hillier SL, et al. Home screening for sexually transmitted diseases in high-risk young women: Randomised controlled trial. Sex Transm Infect 2007; 83:286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cannon CA Piraino AK Golden MR, et al. Sexually transmitted infection testing using online companies: Benefits, drawbacks, and call for official guidance. Sex Transm Dis 2021; 48:e168–e170. [DOI] [PubMed] [Google Scholar]
- 17.McRee AL, Esber A, Reiter PL. Acceptability of home-based chlamydia and gonorrhea testing among a national sample of sexual minority young adults. Perspect Sex Reprod Health 2015; 47:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grandahl M, Larsson M, Herrmann B. ‘To be on the safe side’: A qualitative study regarding users' beliefs and experiences of Internet-based self-sampling for Chlamydia trachomatis and Neisseria gonorrhoeae testing. BMJ Open 2020; 10:e041340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griner SB Vamos CA Puccio JA, et al. “I'll just pick it up…”: Women's acceptability of self-sampling methods for sexually transmitted infection screening. Sex Transm Dis 2019; 46:762–767. [DOI] [PubMed] [Google Scholar]
- 20.VonHoltz LAH Frasso R Golinkoff JM, et al. Internet and social media access among youth experiencing homelessness: Mixed-methods study. J Med Internet Res 2018; 20:e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.United States Census Bureau 2019. Available at: https://www.census.gov/programs-surveys/acs. Accessed June 15, 2021.
- 22.Barbee LA Dhanireddy S Tat SA, et al. Barriers to bacterial sexually transmitted infection testing of HIV-infected men who have sex with men engaged in HIV primary care. Sex Transm Dis 2015; 42:590–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manhart LE, Broad JM, Golden MR. Mycoplasma genitalium: Should we treat and how? Clin Infect Dis 2011; 53 Suppl 3(Suppl 3):S129–S142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.United States Preventive Services Task Force 2016. Available at: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/syphilis-infection-in-nonpregnant-adults-and-adolescents. Accessed June 15, 2021.
- 25.Huppert JS Hesse EA Bernard MC, et al. Accuracy and trust of self-testing for bacterial vaginosis. J Adolesc Health 2012; 51:400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paudyal P Llewellyn C Lau J, et al. Obtaining self-samples to diagnose curable sexually transmitted infections: A systematic review of patients' experiences. PLoS One 2015; 10:e0124310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arias M Jang D Gilchrist J, et al. Ease, comfort, and performance of the HerSwab vaginal self-sampling device for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae. Sex Transm Dis 2016; 43:125–129. [DOI] [PubMed] [Google Scholar]
- 28.Vernon JA Trujillo A Rosenbaum S, et al. Low Health Literacy: Implications for National Health Policy. Mansfield, CT: University of Connecticut, 2007. [Google Scholar]
- 29.Chen X Hay JL Waters EA, et al. Health literacy and use and trust in health information. J Health Commun 2018; 23:724–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barrow RY Ahmed F Bolan GA, et al. Recommendations for providing quality sexually transmitted diseases clinical services, 2020. MMWR Recomm Rep 2020; 68:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]


