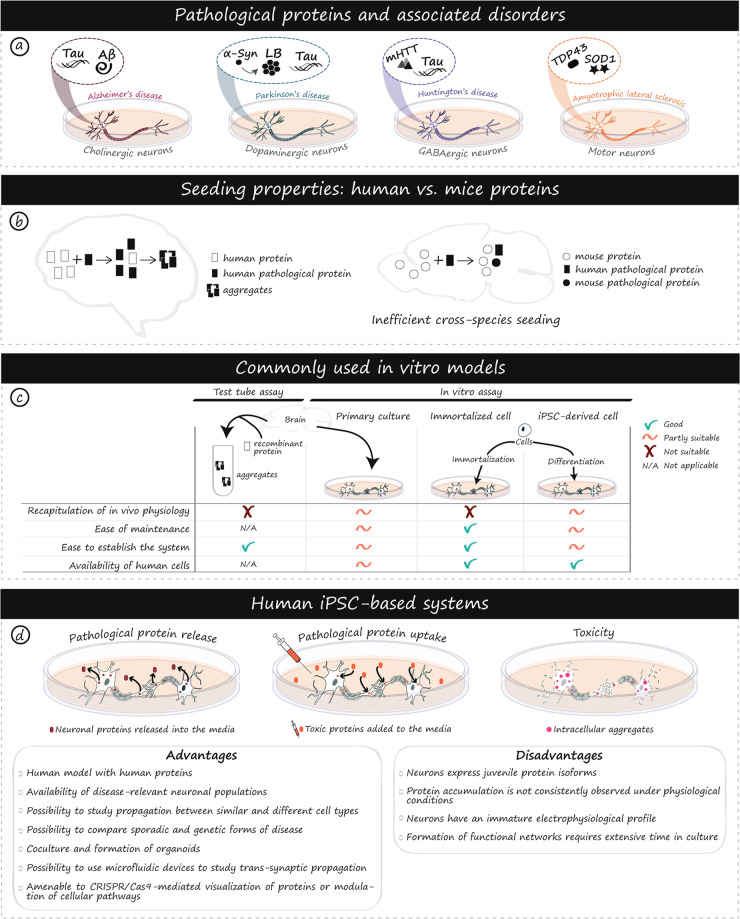
Fig. 1. Prion-like proteins in human diseases and iPSC models.
Protein aggregation is associated with multiple neurodegenerative disorders, involves disease-specific proteins, and affects unique populations of neurons (a). Variability in the propensity to aggregate and inefficient cross-species seeding between human and mouse protein homologs have been observed (b). Ex vivo/test tube and in vitro assays including primary cultures, immortalized cells and iPSC-derived cells are commonly used to study protein propagation. Advantages and disadvantages of each model are listed (c). iPSC-based models enable the production of human neuronal populations of interest that better recapitulate physiological features of human diseases compared to rodent-based models. This unprecedented access to human cells opens new avenues for the study of mechanisms associated with propagation, seeding, and toxicity of pathological proteins (d). As with any disease model, a number of advantages and disadvantages need to be carefully considered to inform and guide experimental choices (d). α-Syn alpha-synuclein, Aβ amyloid beta, AD Alzheimer’s disease, ALS Amyotrophic lateral sclerosis, Cas9 CRISPR associated protein 9, CRISPR Clustered Regularly Interspaced Short Palindromic Repeats, GABA gamma aminobutyric acid, HD Huntington’s disease, LB Lewy body, mHTT mutant huntingtin, PD Parkinson’s disease, SOD1 superoxide dismutase 1, TDP-43 TAR DNA-binding 43 protein.