Abstract
Recent observations suggest that development of venous gas emboli (VGE) during high-altitude flying whilst breathing hyperoxic gas will be reduced by intermittent excursions to moderate altitude. The present study aimed to investigate if an early, single excursion from high to moderate altitude can be used as an in-flight means to reduce high-altitude decompression strain. Ten healthy men were investigated whilst breathing oxygen in a hypobaric chamber under two conditions, once during a 90-min continuous exposure to a simulated cabin altitude of 24,000 ft (High; H) and once during 10 min at 24,000 ft, followed by 30 min at 15,000 ft and by 80 min at 24,000 ft (high–low–high; H–L–H). VGE scores were assessed by cardiac ultrasound, using a 6-graded scale. In H, VGE increased throughout the course of the sojourn at 24,000 ft to attain peak value [median (range)] of 3 (2–4) at min 90, just prior to descent. In H–L–H, median VGE scores were 0 throughout the trial, except for at min 10, just prior to the excursion to 15,000 ft, whence the VGE score was 1.5 (0–3). Thus, an early, single excursion from high to moderate cabin altitude holds promise as an in-flight means to reduce the risk of altitude decompression sickness during long-duration high-altitude flying in aircraft with limited cabin pressurization. Presumably, such excursion acts by facilitating the gas exchange in decompression bubbles from a predomination of nitrogen to that of oxygen.
Keywords: Altitude decompression sickness, Decompression sickness risk, Fighter aircraft, Gas bubble formation, Repeated altitude decompression, VGE
Introduction
Decompression strain remains a cardinal problem in several aerospace settings, for instance, during extra-vehicular space activities (“space walks”), high-altitude high-opening parachuting and, not the least, during high-altitude flying in military aircraft (McLean 2020; Stepanek and Webb 2008). In fighter aircraft, currently operating at peak altitudes ranging between 40,000 and 70,000 ft (≈ 12,200–21,300 m), the cabin-to-ambient pressure differential should not exceed 34.5 kPa (Chaurasiya et al. 2020), to prevent pulmonary overexpansion and rupture, consequent to Boyle gas expansion, in case of a sudden cabin decompression (Bjurstedt 1952; Heimbach and Sheffield 1985; Roth 1968). Thus, at these altitudes, the pilots will be exposed to ambient pressures in between 53 and 39 kPa. Therefore, and since modern fighter aircraft have in-air fueling capacity, permitting extended duration sorties at high altitude, decompression sickness (DCS) in fighter pilots is a growing concern (Westphalen 2020). We have in a series of experiments investigated the decompression strain induced by high-altitude flying (at a cabin pressure corresponding to an altitude of 24,000 ft (7315 m; cabin altitude) interrupted by excursions to cabin altitudes of 15,000–20,000 ft (4572–6096 m), which are realistic for refueling, but not sufficient to completely compress gas bubbles formed in blood and other tissues at high altitude (Ånell et al. 2019, 2020). It appears that under normoxic conditions, such excursions to moderate altitudes increase the decompression strain during subsequent exposure to 24,000 ft, presumably because the increased gas pressure during the excursions serves to replenish body tissue nitrogen (N2) depots and hence increase the degree of N2 supersaturation at high altitude (Ånell et al. 2019, 2020). During hyperoxia, by contrast, i.e. with the pilot breathing 90% oxygen (O2), repeated excursions to moderate altitude [18,000 ft (5486 m)] seemed to reduce the decompression strain at high altitude (24,000 ft); an effect that was attributed to facilitated gas exchange in the decompression-induced bubbles, from a predomination of N2 to that of O2 (Ånell et al. 2021). Notwithstanding, occasional incidences of DCS occurred during the 30 min at 24,000 ft, prior to the first excursion to moderate altitude (Ånell et al. 2021). In addition, it was not clear whether iterative excursions were needed to accomplish sustained reduction of the decompression strain at high altitude (Ånell et al. 2021). The aim of the present study was to further explore the possibility to use an early, single excursion from high to moderate altitude as an in-flight means to reduce the risk of altitude DCS. We hypothesised that, whilst breathing 100% O2, a 30-min excursion to 15,000 ft after 10 min at 24,000 ft, will reduce the decompression strain to acceptable levels during a subsequent 80-min exposure to 24,000 ft, and that the decompression strain during such time-altitude profile will be markedly lower than during a continuous 90-min exposure to 24,000 ft.
Methods
Subjects
Ten healthy non-smoking men volunteered as test subjects. Their mean (range) age and body mass index were 44.6 (20–54) years and 26.8 (22.0–34.1) kg/m2, respectively. The subjects were recruited among divers (n = 8) and medical students (n = 2). All were familiar with hypobaric chamber exposures and informed about the symptoms of DCS. One of the divers had on a previous occasion experienced DCS (type 1) in the hypobaric chamber with total remission of symptoms during the recompression phase of the exposure. The divers had an approved yearly examination for duty before the exposures and the remaining two subjects were examined by a flight surgeon prior to admission. Before giving their consent to participate, the subjects were informed that participation was voluntary and could be terminated at any given time. The experimental procedures and protocol were approved by the Swedish National Ethics Review Authority (Approval # 2021-00661).
Equipment
All experiments were performed in a 21-m3 hypobaric chamber (AB Motala Verkstad. 1953, Reg. nr: 20621) at the Division of Environmental Physiology, KTH Royal Institute of Technology, Stockholm. The experiments were monitored and recorded using a video/audio system (JVC MI 5000 Victor Company, Tokyo, Japan). Each subject was breathing O2 from a standard 50-l bottle for compressed gas (max filling pressure: 200 bar), connected via a pressure-reduction valve to a pressure-demand full facemask regulator (Atmosphere, Poseidon Diving Systems AB, Göteborg, Sweden). To avoid high levels of O2 in the chamber, the expiratory side of each subject’s facemask was, via a respiratory hose (diameter 38 mm), connected to a confined space in the chamber, that was regularly ventilated throughout the exposure. In addition, the hypobaric chamber was intermittently ventilated during the course of each experiment.
VGE was detected in the right cardiac ventricle from ultrasound four-chamber cardiac images, using a phased-array transducer (1–5 MHz) and an ultrasound system (CX50, Philips Ultrasound Bothell, WA, USA). The prevalence of VGE was evaluated using the 6-graded Eftedal–Brubakk (EB) scale (0–5): 0 = no visible bubbles; 1 = occasional bubbles; 2 = at least one new bubble every fourth heartbeat; 3 = at least one new bubble every heartbeat; 4 = at least one bubble/cm2; 5 = “white out”, single bubbles cannot be discriminated (Eftedal et al. 2007; Nishi et al. 2003). All cardiac ultrasound videos/images were stored on a computer hard drive (Latitude E5530, Dell, USA). Cardiac Output (CO) and heart rate (HR) were measured using impedance technique (Physioflow PF07 system, Enduro, Manatec Biomedical, Paris, France) and data were stored on a computer (Latitude E5530, Dell, USA). Capillary oxyhaemoglobin saturation (SpO2) was monitored continuously by a pulse oximeter with the sensor placed on an index finger. (Radical 7 Monitor MASIMO SET, Rainbow CA, USA). O2 content in the chamber gas was monitored using an oxygen sensor (Datex Normocap 200 Oxy, Datex Finland). SpO2 and chamber O2 signals were registered using a data acquisition system (BioPac MP150, BioPac Systems Inc., USA) in combination with a computer (Latitude E5530, Dell, USA).
Procedures
Each subject was examined on two separate occasions, interspersed by ≥ 72 h; on one occasion, the simulated altitude exposure was 90 min continuously at 24,000 ft (condition High, H) and on the other occasion, the 90-min stay at 24,000 ft was interrupted after 10 min by a 30-min excursion to 15,000 ft (high–low–high, H–L–H). The order of the two trials was alternated among subjects in a counter-balanced fashion. For the individual subject, the two trials were performed at approximately the same time of the day.
All hypobaric exposures were performed with an inside experimenter accompanying the subjects in the chamber. The subjects were instructed to avoid heavy exercise (48 h) and nicotine intake (4 h) before an exposure. Immediately prior to each experiment, the subject, who was dressed in shorts and sneakers, performed 150 unloaded squats during a 10-min period, in an attempt to equalize the amount of micronuclei in the circulation (Dervay et al. 2002). Thereafter, he was instrumented with six pre-gelled electrodes on the thorax and neck for impedance- and electro-cardiography recordings. Throughout each experiment, the subject was lying horizontally on his left side on a gurney. The subject donned the facemask upon initiation of ascent and then breathed O2 throughout the experiment, whereas the inside experimenter breathed O2 during the experiment as well as 1 h prior to ascent. Ascent and descent rates were 5000 ft/min. HR, CO, SpO2 and chamber O2 content were recorded continuously throughout each experiment, whereas the prevalence of VGE was determined intermittently, at 5000, 10,000, 15,000 and 20,000 ft during ascent and descent as well as every 5th minute at the plateau altitudes. In conjunction with all VGE determinations during ascent and descent, and also with every third determination during the altitude plateau phases, the subject performed three unloaded knee bends/extensions while in the left decubitus position. This was done to provoke release of bubbles attached to vascular endothelium into the stream of mixed venous blood (Foster and Butler 2009; Gennser et al. 2012; Jankowski et al. 2004). An exception from the 15-min interval between knee-bend provocations was the initial period at 24,000 ft in the H–L–H condition, during which a knee-bend provocation was performed after 10 min, just prior to the descent to 15,000 ft. In conjunction with each VGE determination, the subject was also asked about symptoms of DCS (discomfort/pain) and, if any, to rate their severity using a ratio scale (Borg 1982). DCS was not the primary endpoint of the experiment but any exposure was a priori set to be terminated if a subject reported DCS symptoms or the inside experimenter graded a persistent EB-score > 3.
Statistical analyses
VGE scores are ordinal data and were converted using a rank-transformation described by Baguley (2012). The VGE scores after transformation, as well as the continuous variables (HR and CO), were analysed with a one-way analysis of variance (ANOVA). p < 0.05 was considered significant for all test variables.
Results
The experiments were conducted without any DCS incidences or adverse events. In both trials, the rates of ascent and descent were kept close to the stipulated values, with changes in chamber altitude being attained within 45 s from the expected time.
Venous gas emboli (VGE)
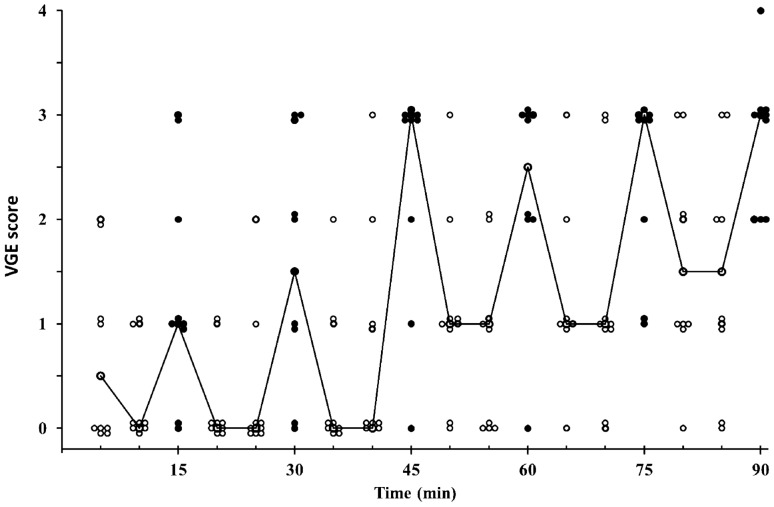
In the H condition, the presence of VGE increased during the course of the exposure to 24,000 ft (p < 0.001), with the highest values being noted at the end of the exposure, both at rest [at 85 min, median (range): 1.5 (0–3)] and after knee bends [90 min: 3 (2–4)] (Fig. 1). In the H–L–H condition, the highest VGE score was noted during knee bends after 10 min at 24,000 ft [1.5 (0–3)]. During the excursion to 15,000 ft and throughout the subsequent 80 min at 24,000 ft, median VGE scores were 0 (0–3) in the H–L–H condition (Fig. 2); there was a tendency, although not statistically significant (p = 0.24), of occasional bubbles reoccurring during the latter part of the 80-min post-excursion period at 24,000 ft in H–L–H (Fig. 2).
Fig. 1.
Individual and median (line) venous gas emboli (VGE) scores in Condition H (i.e. during 90 min at 24,000 ft) at rest (open circles) and following knee-bends (filled circles); n = 10. Thus, at each time point, a circle represents either the value of one individual (n = 10) or the median value (interconnected by lines)
Fig. 2.
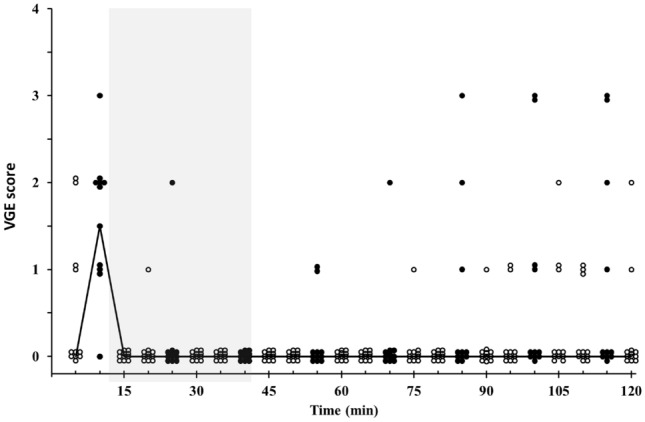
Individual and median (line) venous gas emboli (VGE) scores in Condition H–L–H, i.e. during 10 min at 24,000 ft, followed by 30 min at 15,000 ft (shaded area) and finally by 80 min at 24,000 ft. Values are at rest (open circles) and following knee-bends (filled circles); n = 10. Thus, at each time point, a circle represents either the value of one individual (n = 10) or the median value (interconnected by lines)
The overall prevalence of VGE during the 90 min at 24,000 ft, was markedly lower in the H–L–H than in the H condition (F(1,37) = 18.78, p < 0.001) (Figs. 1, 2). This difference was solely attributable to the last 80 min at 24,000 ft—i.e. to the post-excursion period in the H–L–H condition—with lower VGE in H–L–H than H, both at rest (F(1,19) = 53.3, p < 0.001) and following knee bends (F(1,19) = 32.77, p < 0.001) during this period (Figs. 1, 2). Whereas, during the initial period at 24,000 ft (prior to the excursion to 15,000 ft in the H–L–H condition), VGE scores were similar in H and H–L–H, both at rest and after the knee-bend provocation (which, was undertaken after 10 min at 24,000 ft in H–L–H and after 15 min in H) (Figs. 1, 2).
During the initial ascent from sea level to 24,000 ft, VGE scores were 0 at all stops (5000, 10,000, 15,000 and 20,000 ft) in both the H and H–L–H trial, whereas during the final descent, VGE was higher (p ≤ 0.05) in the H than in the H–L–H condition at altitude stops > 5000 ft (Table 1).
Table 1.
Venous gas emboli scores during step-wise initial ascent from sea level to 24,000 ft and final descent from 24,000 ft to sea level, in conditions H and H–L–H
| Ascent | Descent | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sea level (pre) | 5 kft | 10 kft | 15 kft | 20 kft | 24 kft | 24 kft | 20 kft | 15 kft | 10 kft | 0.5 kft | Sea level (post) | |
| H rest | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.5 (0–3) | 1 (0–3) | 0 (0–3) | 0 (0–3) | 0 (0–3) | 0 (0–3) |
| H knee-bends | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0–1) | 3 (0–4) | 3 (0–4) | 1.5 (0–4) | 0.5 (0–4) | 0 (0–3) | 0 (0–3) |
| H–L–H rest | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0–2) | 0 (0–3) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) |
| H–L–H knee-bends | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0–1) | 0 (0–3) | 0 (0–3) | 0 (0–3) | 0 (0–3) | 0 (0–3) | 0 (0–3) |
Values are medians (range); n = 10
Cardiac output (CO), heart rate (HR) and capillary oxyhaemoglobin saturation (SpO2)
In both conditions, CO and HR dropped substantially during the initial 15 min of the trial, and then remained relatively stable throughout the remaining course of the trial (Table 2). There was no difference in CO or HR between the H and H–L–H condition, at any given time of the trials (Table 2). In both conditions, SpO2 remained high (> 95%) at all times for all participants.
Table 2.
Cardiac output (CO) and heart rate (HR) during the time at altitude in conditions H and H–L–H (24,000 or 15,000 ft). Values are means ± SD; n = 10
| Variable | Time (min) | 5 | 25 | 40 | 55 | 70 | 85 | 100 | 115 |
|---|---|---|---|---|---|---|---|---|---|
| CO (l/min) | H | 7.5 ± 0.2 | 5.7 ± 0.9 | 5.5 ± 0.9 | 5.3 ± 0.7 | 5.4 ± 1.3 | 5.4 ± 1.8 | – | – |
| H–L–H | 7.0 ± 3.0 | 5.9 ± 1.7 | 5.5 ± 1.5 | 5.6 ± 1.4 | 5.4 ± 1.5 | 5.1 ± 1.4 | 5.1 ± 1.6 | 5.0 ± 1.5 | |
| HR (beats/min) | H | 76 ± 8 | 66 ± 10 | 66 ± 11 | 64 ± 9 | 66 ± 10 | 62 ± 10 | – | – |
| H–L–H | 76 ± 7 | 63 ± 9 | 66 ± 10 | 64 ± 8 | 63 ± 11 | 62 ± 11 | 62 ± 10 | 62 ± 10 |
Discussion
The aim was to investigate whether, and to what degree, a single, early excursion from high to moderate altitude will decrease decompression strain, as indicated by reduced vascular bubble formation, during a subsequent prolonged exposure to high altitude. The results demonstrated that the ascents from sea level to 24,000 ft induced rapid formation of decompression bubbles, with similar presence of VGE in the H and H–L–H trials, during the initial knee-bend provocation after 10–15 min at altitude. By contrast, during the remaining 80 min at 24,000 ft, i.e. in the H–L–H condition, following a 30-min excursion to 15,000 ft, the occurrence of VGE was considerably less in the H–L–H than the H condition, in particular during the knee-bend provocations. There were no DCS incidences in any of the present experiments.
Our findings that long-term uninterrupted exposure to 24,000 ft whilst breathing a hyperoxic gas is associated with substantial and prolonged decompression strain, as indicated by the high VGE levels, is in accordance with previous reports (Ånell et al. 2021; Foster et al. 1998; Pilmanis et al. 2005; Webb et al. 1998; Webb and Pilmanis 1993). It thus appears that there is little benefit from breathing a gas mixture containing 90–100% O2 compared to a normoxic gas as regards the prevalence of VGE during the initial 90 min at altitudes exceeding 22,500 ft (Ånell et al. 2021; Pilmanis et al. 2005). Since decompression-dependent de novo formation of gas bubbles requires a very pronounced supersaturation of gas molecules, in blood corresponding to a pressure drop of about 110 ATA (Doolette 2009), it is generally accepted that precursors are needed to induce formation of decompression bubbles in human tissues (Doolette 2009; Vann et al. 1980; Yount 1976). Thus, the prevailing notion is that during acute exposure to altitude, VGE are formed from pre-existing gaseous micronuclei (for review, see Stepanek and Webb 2008). It must be presumed that, in the present experiments, the gas phase of such micronuclei was predominantly consisting of nitrogen (N2). Despite that the subjects were breathing O2 during the 5-min ascent and onwards, it is also likely that the initial bubble growth upon arrival at 24,000 ft was mainly due to inward diffusion of N2 molecules, as a result of N2 supersaturation in the venous blood stream and adjacent tissues. Thus, a rapid ascent from sea level to 24,000 ft whilst breathing a normoxic O2/N2 mixture, induced a whole-body washout of N2, from mixed venous blood via the pulmonary ventilation, that occurred at an exponentially decaying rate with a half time of about 45 min (Ånell et al. 2020). Despite a 40% higher inspired partial pressure of O2 in the present experiments, it is likely that N2 remained a salient component in the majority of the capillary beds and hence in mixed venous blood for more than 30 min at 24,000 ft. In fact, there is evidence to suggest that, even during O2 breathing, DCS and the growth of decompression VGE are to large extent attributable to diffusion of N2 molecules from tissue compartments with half-times for N2 washout exceeding 90 min (Conkin et al. 1987; Lundin 1960). Prolonged O2 breathing at 24,000 ft, will eventually, by way of diffusion, change the gas content in the decompression bubbles to O2, whereafter the bubbles will shrink, become unstable and disappear due to outward diffusion of O2 in locations where metabolism has created low PO2 in the bubble surroundings (Blogg et al. 2003; Hyldegaard and Madsen 1989, 2007). Judging by experiments in rodents, a noticeable reduction of bubble size following a switch from breathing air to O2 at altitude, may take considerable time (≫ 1 h), at least in tissues with slow washout rates of N2, as for instance in adipose tissue (Hyldegaard and Madsen 1989, 2007). In fact, a switch from air to O2 at altitude, may initially lead to growth of air bubbles injected in adipose tissue, presumably due to the greater transport capacity of O2 than N2 in blood, in combination with increasing diffusion gradient for N2, as the share of O2 molecules in the bubbles increases (Hyldegaard et al. 2001).
Even though there were no DCS incidents in the present experiments, the VGE results support the notion that a mere shift of the breathing gas from normoxic to pure O2 (or a hyperoxic mixture) whilst flying, is not sufficient to prevent DCS during prolonged exposure to high altitude (Ånell et al. 2021; Pilmanis et al. 2005). Pre-oxygenation protocols to wash out inert gases prior to the flight sortie is an efficient means to reduce DCS risk at high altitude (Pilmanis et al. 2005; Stepanek and Webb 2008), but is very time consuming (1–6 h) and cumbersome to undertake and hence is typically not practised by the pilots. Pre-flight whole-body vibration may be an alternative, less time-consuming means to reduce in-flight decompression strain (Elia et al. 2021); presumably such vibration preconditioning acts by reducing the amount of micronuclei precursors, although its efficacy in reducing DCS symptoms remains to be settled (Elia et al. 2021).
Our findings that in the H–L–H condition the prevalence of VGE was low throughout the altitude exposure and markedly lower in the H–L–H than H condition confirm our initial hypotheses and suggest that an alternative means to reduce decompression strain during long-duration flying at high altitude, may be an early, transient excursion to moderate cabin altitude whilst breathing a hyperoxic gas. As outlined previously (Ånell et al. 2021), we assume that the VGE-reducing mechanism of such procedure is a facilitated exchange of the gas content in the decompression bubbles from N2 to O2 domination. Thus, although the Boyle compression resulting from an altitude transition from 24,000 to 15,000 ft does not suffice to demolish decompression bubbles (Ånell et al. 2020), it will reduce their diameters by about 12%, and the associated increase in bubble surface tension and consequent 12% increase in intra-bubble pressure, as dictated by the Young–Laplace equation, will facilitate outward diffusion of N2 from the bubbles. In addition, the increase in total pressure, from 39 kPa at 24,000 ft to 57 kPa at 15,000 ft, during oxygen breathing will, according to Fick’s first law, accentuate the diffusion gradients for N2 and O2, by increasing the local partial pressures of these gases on the inside and outside, respectively, of the bubble membranes. Thus, the cabin-altitude excursion will accelerate the exchange of bubble content to O2 and hence decrease the VGE development upon the subsequent period at 24,000 ft. In addition, it cannot be ruled out that the early hyperoxic compression may have destabilized the decompression bubbles by interrupting any surface recoating towards a greater share of the bubble membranes consisting of hydrophobic proteins or other surface tension reducing molecules (Harvey et al. 1944; Swan et al. 2014).
The prevalence of VGE dropped promptly upon the excursion descent to 15,000 ft, with virtually no VGE during the knee-bend provocation after 15 min at this altitude (Fig. 2). To what extent this reflected a rapid intra-bubble gas exchange, towards an increased share of O2, remains to be settled. In part, compression reduction of bubble size might have contributed to the scarcity of VGE at 15,000 ft, despite a mere 12% estimated reduction of bubble radii from Boyle compression. Thus, even such relatively discrete reductions of bubble radii might render a share of the bubbles smaller than the detection limit of the ultrasound system (i.e. < 20–30 μm). That also a normoxic compression from 24,000 to 15,000 ft substantially reduced the prevalence of VGE (Ånell et al. 2021) suggests that such compression effect on VGE occurrence may not have been negligible in the present H–L–H experiments. On the other hand, that the VGE scores remained low upon re-ascent to 24,000 ft in the H–L–H condition suggests that compression alone could not explain the low VGE prevalence during the latter part of the excursion to 15,000 ft; during normoxia, re-ascent from 15,000 to 24,000 ft resulted in a prompt increase in VGE occurrence (Ånell et al. 2020). A factor that likely contributed to faster shrinkage and collapse of decompression bubbles during the present excursion to 15,000 ft is the increased “O2 window” induced by the elevation of ambient pressure. The synonymic terms “oxygen window” (Behnke 1967) and “inherent unsaturation” (Hills 1977) refer to the principle that the sum of alveolar and hence arterial partial pressures of gas exceeds the sum of gas tensions in the tissues, because of the oxygen metabolism in the tissues (Behnke 1967; Hills, 1977). Thus, at any given level of metabolic rate, provided that alveolar PO2 is sufficiently high to saturate haemoglobin with O2 during its lung passage, which, judging by the SpO2 values, was always the case in the present experiments, then the oxygen window will increase as a function of increased alveolar PO2 (Tikuisis and Gerth 2003; Van Liew et al. 1993). Even though the effects of the O2 window on decompression strain are only well established for steady-state conditions (Tikuisis and Gerth 2003), which were probably not attained during the excursion to 15,000 ft, the increased O2 window during this period likely facilitated shrinkage of decompression bubbles. Thus, both theoretical analyses and experimental evidence suggest that an increased oxygen window will shrink decompression bubbles irrespective of the share of inert gas in the bubbles (Foster et al. 1998; Randsoe and Hyldegaard 2012; Van Liew et al. 1993).
Taken together, the present and previous studies (Ånell et al. 2019, 2020, 2021; Pilmanis et al. 2002) suggest that pressure increments induced by excursions from high (24,000–25,000 ft) to low (≤ 900 ft) altitudes will suffice to completely compress decompression bubbles, resulting in sustained (≥ 30 min) very low VGE scores during the subsequent exposure to high altitude, even if the pilot/subject is breathing normoxic gas during the excursions (Ånell et al. 2019; Pilmanis et al. 2005). By contrast, excursions from high (24,000 ft) to moderate (15,000–18,000 ft) altitude will not demolish decompression bubbles by way of compression, but will lead to sustained (> 30 min) very low VGE scores during the subsequent exposure to high altitude, if the pilot/subject is breathing a hyperoxic gas (Ånell et al. 2021), but not if he/she is breathing a normoxic gas (Ånell et al. 2019, 2020).
In the H–L–H condition, after about 40 min at 24,000 ft, following the excursion, there was a tendency (albeit not statistically significant) for partial reappearance, of VGE (Fig. 2). This may reflect the aforementioned N2 supersaturation in tissue compartments with slow N2 washout. Thus, the net effect on VGE prevalence during a longer period at 24,000 ft (> 80 min) following an excursion to 15,000 ft, with influence of the prolonged O2 breathing opposing that of the slow N2 washout compartments, remains to be established.
Even though present findings suggest that an early, single excursion/bounce to moderate cabin altitude holds promise as an efficient means to ad hoc reduce decompression stress and hence prolong a fighter pilot’s operational time at high altitude, it should be noted that the study needs to be expanded before implementing such strategy in practice. Thus, although VGE determinations are valid markers of decompression strain (Conkin et al. 1998; Eftedal et al. 2007), accounts of a causal relationship between VGE prevalence and DCS are rare (Ljubkovic et al. 2010). A substantially larger subject group needs to be investigated to establish the effect of an early excursion on the risk of developing DCS at high altitude. In addition, the protocol needs to be refined, establishing the optimal duration and altitude of the excursion as well as whether a slightly lower O2 content in the breathing gas, as expected in aircraft with on-board oxygen generating systems (OBOGS), would be as effective as pure O2.
Finally, it should be mentioned that the present technique to reduce high-altitude decompression strain by undertaking an early excursion to moderate altitude might be used not merely by fighter pilots but also in high-altitude parachute operations, during which particularly the jump-master appears to be at risk of suffering DCS (Ånell et al. 2019).
Author contributions
All authors designed the study and participated in the data collection. RÅ analysed the data. RÅ and OE drafted the manuscript. All authors revised the manuscript and approved its final version.
Funding
Open access funding provided by Royal Institute of Technology. This study was supported by a grant from the Swedish Armed Forces (No 922: 0905).
Declarations
Conflict of interest
The authors have no conflict of interest to declare.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that all human research participants involved in this study provided informed consent for publication of the data contained herein.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ånell R, Grönkvist M, Eiken O, Gennser M. Nitrogen washout and venous gas emboli during sustained vs. discontinuous high-altitude exposures. Aerosp Med Hum Perform. 2019;90(6):524–530. doi: 10.3357/AMHP.5207.2019. [DOI] [PubMed] [Google Scholar]
- Ånell R, Grönkvist M, Gennser M, Eiken O. Evolution and preservation of venous gas emboli at alternating high and moderate altitude exposures. Aerosp Med Hum Perform. 2020;91(1):11–17. doi: 10.3357/AMHP.5447.2020. [DOI] [PubMed] [Google Scholar]
- Ånell R, Grönkvist M, Gennser M, Eiken O. Hyperoxic effects on decompression strain during alternating high and moderate altitude exposures. Aerosp Med Hum Perform. 2021;92(4):223–230. doi: 10.3357/AMHP.5707.2021. [DOI] [PubMed] [Google Scholar]
- Baguley T. Serious Stats. A guide to advanced statistics for the behavioural sciences. Basingstoke: Palgrave Macmillan; 2012. [Google Scholar]
- Behnke AR (1967) The isobaric (oxygen window) principle of decompression. In: The new thust seaward: transactions of the third marine technology society conference and exhibit. Marine Technology Society, San Diego
- Bjurstedt H (1952) Explosiv dekompression under flygning samt åtgärder för att motverka dess skadliga inverkningar. Kungl Krigsvet. Akad. Handl. 3:1–25
- Blogg SL, Gennser M, Loveman GAM, Seddon FM, Thacker JC, White MG. The effect of breathing hyperoxic gas during simulated submarine escape on venous gas emboli and decompression illness. Undersea Hyperb Med. 2003;30(3):163–174. [PubMed] [Google Scholar]
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- Chaurasiya KL, Bhattacharya B, Varma AK, Rastogi S. Dynamic modeling of a cabin pressure control system. J Aerosp Eng. 2020;234(2):401–415. doi: 10.1177/0954410019867578. [DOI] [Google Scholar]
- Conkin J, Edwards BF, Waligora JM, Horrigan Jr DJ (1987) Empirical models for use in designing decompression procedures for space operations. NASA Technical Memorandum, NASA, Houston
- Conkin J, Powell MR, Foster PP, Waligora JM. Information about venous gas emboli improves predicition of hypobaric decompression sickness. Aviat Space Environ Med. 1998;69:8–16. [PubMed] [Google Scholar]
- Dervay J, Powell M, Butler B, Fife C. The effect of exercise and rest duration on the generation of venous gas bubbles at altitude. Aviat Space Environ Med. 2002;73:22–27. [PubMed] [Google Scholar]
- Doolette DJ. Haldane still rules! In: Lang MA, Brubakk AO, editors. The future of diving: 100 years of Haldane and beyond. Washington DC: Smithsonian Institution, Scholary Press; 2009. pp. 29–32. [Google Scholar]
- Eftedal O, Lydersen S, Brubakk A. The relationship between venous gas bubbles and adverse effects of decompression after air dives. Undersea Hyperb Med. 2007;34(2):99–105. [PubMed] [Google Scholar]
- Elia A, Eiken O, Ånell R, Grönkvist M, Gennser M. Whole body vibration preconditioning reduces the formation and delays the manifestation of high-altitude-induced venous gas emboli. Exp Physiol. 2021;106:1743–1751. doi: 10.1113/EP089522. [DOI] [PubMed] [Google Scholar]
- Foster PP, Butler BD. Decompression to altitude: assumptions, experimental evidence and future directions. J Appl Physiol. 2009;106(2):678–690. doi: 10.1152/japplphysiol.91099.2008. [DOI] [PubMed] [Google Scholar]
- Foster PP, Conkin J, Powell MR, Waligora JM, Chhikara RS. Role of metabolic gases in bubble formation during hypobaric exposures. J Appl Physiol. 1998;84(3):1088–1095. doi: 10.1152/jappl.1998.84.3.1088. [DOI] [PubMed] [Google Scholar]
- Gennser M, Jurd KM, Blogg SL. Pre-dive exercise and post-dive evolution of venous gas emboli. Aviat Space Environ Med. 2012;83(1):30–34. doi: 10.3357/ASEM.2893.2012. [DOI] [PubMed] [Google Scholar]
- Harvey EN, Barnes DK, McElroy WD, Whiteley AH, Pease DC, Cooper KW. Bubble formation in animals. J Cell Comp Phys. 1944;24(1):1–22. doi: 10.1002/jcp.1030240102. [DOI] [Google Scholar]
- Heimbach RD, Sheffield PJ. Protection in the pressure environment: cabin pressurization and oxygen equipment. In: DeHart RL, editor. Fundamentals of aerospace medicine. Philadelphia: Lea and Febiger; 1985. [Google Scholar]
- Hills BA. Decompression sickness: the biophysical basis of prevention and treatment. New York: Wiley; 1977. [Google Scholar]
- Hyldegaard O, Madsen J. Influence of heliox, oxygen and N2O–O2 breathing on N2 bubbles in adipose tissue. Undersea Biomed Res. 1989;16:185–193. [PubMed] [Google Scholar]
- Hyldegaard O, Madsen J. Effect of hypobaric air, oxygen, heliox (50:50), or heliox (80:20) breathing on air bubbles in adipose tissue. J Appl Physiol. 2007;103:757–762. doi: 10.1152/japplphysiol.00155.2007. [DOI] [PubMed] [Google Scholar]
- Hyldegaard O, Kerem D, Melamed Y. Effect of combined recompression and air, oxygen, or heliox breathing on air bubbles in rat tissues. J Appl Physiol. 2001;90:1639–1647. doi: 10.1152/jappl.2001.90.5.1639. [DOI] [PubMed] [Google Scholar]
- Jankowski LW, Tikusis P, Nishi RY. Exercise effects during diving and decompression on postdive venous gas emboli. Aviat Space Environ Med. 2004;75:489–495. [PubMed] [Google Scholar]
- Ljubkovic M, Marinovic J, Obad A, Breskovis T, Brubakk AO, Gaustad SE, Duijic Z. Consecutive trimix diving is associated with high incidence of gas bubbles arterialization. FASEB J. 2010;24(S1):803.2. doi: 10.1096/fasebj.24.1_supplement.803.2. [DOI] [Google Scholar]
- Lundin G. Nitrogen elimination from the tissues during oxygen breathing and its relationship to the fat: muscle ratio and the localization of bends. J Physiol. 1960;152:167–175. doi: 10.1113/jphysiol.1960.sp006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean WM. Reducing the Risk of Altitude Decompression Sickness. Lynchburg J Med Sci. 2020;2(2):Art. 16. [Google Scholar]
- Nishi RY, Brubakk AO, Eftedal O. Bubble detection. In: Brubakk AO, Neuman TS, editors. Bennett and Elliott’s physiology and medicine of diving. Edinburgh: Saunders, Elsevier Science Ltd; 2003. pp. 501–529. [Google Scholar]
- Pilmanis AA, Webb J, Kannan N, Balldin U. The effect of repeated altitude exposures on the incidence of decompression sickness. Aviat Space Environ Med. 2002;73:525–531. [PubMed] [Google Scholar]
- Pilmanis AA, Webb JT, Balldin UI. Partial pressure of nitrogen in breathing mixtures and risk of altitude decompression sickness. Aviat Space Environ Med. 2005;76:635–641. [PubMed] [Google Scholar]
- Randsoe T, Hyldegaard O. Effects of oxygen breathing on micro oxygen bubbles in nitrogen-depleted rat adipose tissue at sea level and 25 kPa altitude exposures. J Appl Physiol. 2012;113:426–433. doi: 10.1152/japplphysiol.00193.2012. [DOI] [PubMed] [Google Scholar]
- Roth EM (1968) Rapid (explosive) decompression emergencies in pressure-suited subjects. NASA CR-1223. Produced by The Lovelace Foundation for Medical Education and Research, Albuquerque [PubMed]
- Stepanek J, Webb J. Physilology in decompressive stress. In: Davis J, Johnson R, Stepanek J, Fogarty J, editors. Fundamentals of aerospace medicine. Philadelphia: . Lippincott, Williams & Wilkins; 2008. [Google Scholar]
- Swan J, Wilbur J, Moodie K, Kane S, Knaus D, Phillips S, Beach T, Fellows A, Magari P, Buckey J. Microbubbles are detected prior to larger bubbles following decompression. J Appl Physiol. 2014;116:790–796. doi: 10.1152/japplphysiol.01156.2013. [DOI] [PubMed] [Google Scholar]
- Tikuisis P, Gerth WA. Decompression theory. In: Brubakk AO, Neuman TS, editors. Bennet and Elliots physiology and medicine of diving. 5. Oxford: Elseviers Science Limited; 2003. pp. 419–454. [Google Scholar]
- Van Liew HD, Conkin J, Burkard ME. The oxygen window and decompression bubbles: estimates and significance. Aviat Space Environ Med. 1993;64:859–865. [PubMed] [Google Scholar]
- Vann RD, Grimstad J, Nielsen CH. Evidence for gas nuclei in decompressed rats. Undersea Biomed Res. 1980;7(2):107–112. [PubMed] [Google Scholar]
- Webb JT, Pilmanis AA. Breathing 100% oxygen compared with 50% oxygen: 50% nitrogen reduces altitude-induced venous gas emboli. Aviat Space Environ Med. 1993;64(9, Pt 1):808–812. [PubMed] [Google Scholar]
- Webb JT, Pilmanis AA, O’Connor RB. An abrupt zero-preoxygenation altitude treshold for decompression sickness symptoms. Aviat Space Environ Med. 1998;69(4):335–340. [PubMed] [Google Scholar]
- Westphalen N. Military medicine capabilities in the Australian Defence Force. J Mil Med Vet Health. 2020;28(2):39–49. [Google Scholar]
- Yount DE. Bubble formation in gelatin: a model for decompression sickness. J Appl Phys. 1976;47(11):5081–5089. doi: 10.1063/1.322469. [DOI] [Google Scholar]