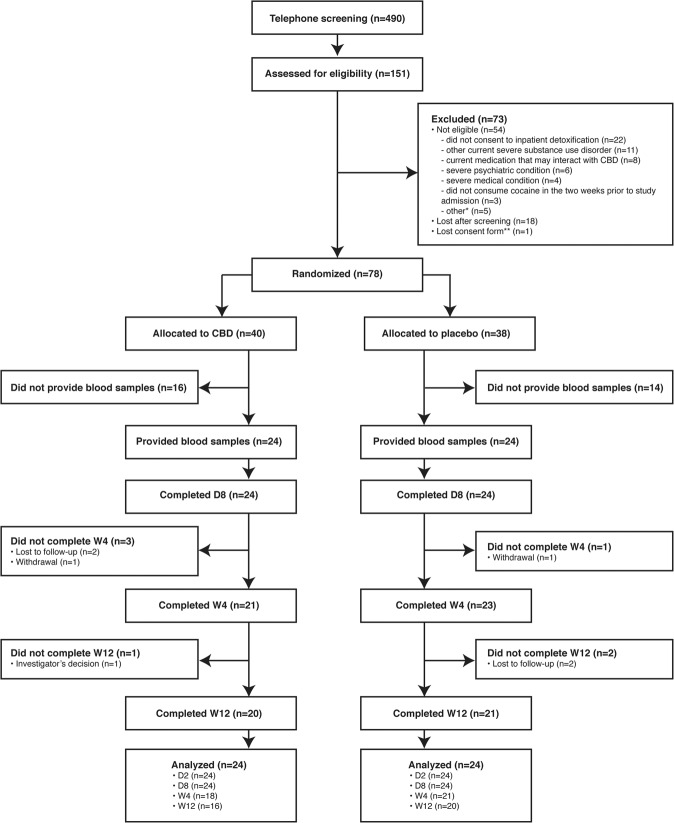
Fig. 1. Consolidated Standards of Reporting Trials (CONSORT) flowchart of participants (adapted with permission from Mongeau-Pérusse et al. [33]).
*Other reasons for exclusion were male fertility issues (n = 2), immunosuppression (n = 2), and not currently moderate or severe CUD according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria (n = 1). **Lost consent form and refusal to reconsent ended participation. The data safety and monitoring board requested that no data from this participant be used in the study. CBD cannabidiol, D day, n number of participants, W week.