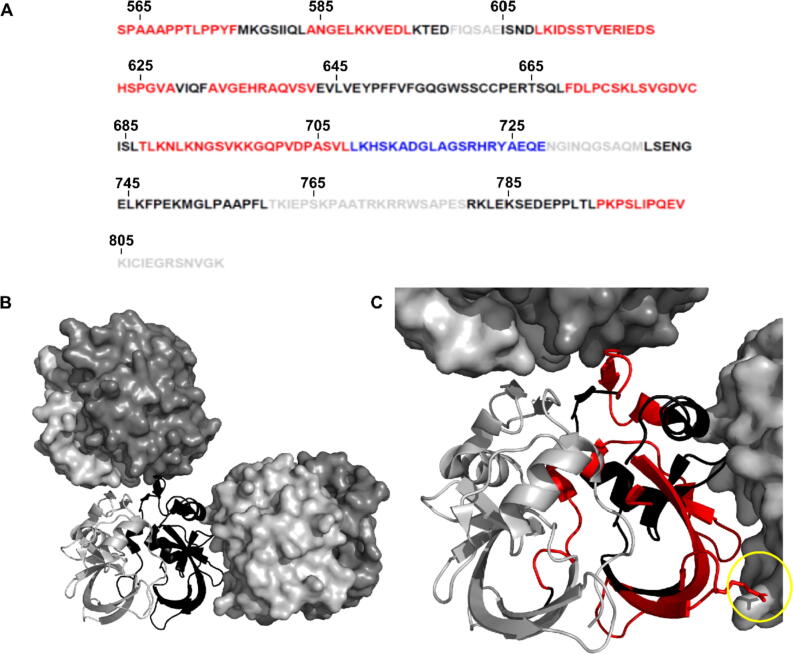
Figure 4.
Mapping of HD exchange in the presence of 14-3-3ζ, on the sequence of the Ataxin-1 AXH-C construct and the structure of its AXH domain. (A) Amino acid sequence of the AXH-C construct (Ataxin-1 residues 563–816). Red = increased HD exchange in the 14-3-3ζ/AXH-C complex vs AXH-C. Blue = decreased HD exchange in complex vs AHX-C. Black = no change in HD exchange. Grey = no data available. (B) Crystal structure of the AXH domain (Ataxin-1 residues 567–689, PDB ID 4APT), in the absence of 14-3-3ζ. An AXH dimer is shown in cartoon representation with one monomer shown in grey and the other in black. Two other dimers related by crystallographic symmetry are also shown in surface representations. (C) This panel zooms in on panel B, with amino acids with increased HDX exchange (p < 0.001) in the presence of 14-3-3ζ, shown in red on one the AXH molecules (i.e. the one in black cartoon representation). These regions are clearly present in the AXH dimer interface, as well as the interfaces with the two dimers related by crystallographic symmetry, suggesting these regions become more solvent exposed upon the interaction of AXH-C with 14-3-3ζ. The yellow circle highlights residue R638 on the AXH domain, which has been suggested to play a role in Ataxin-1 self-association and fibril formation.46