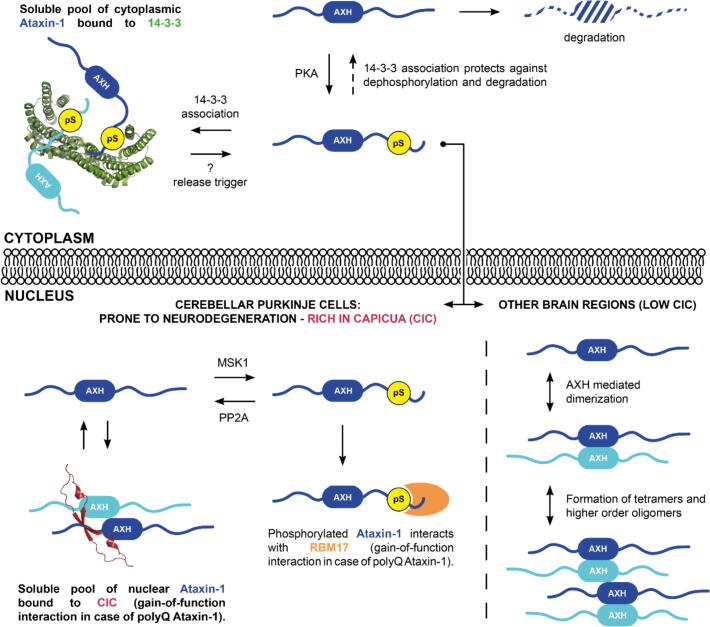
Figure 8.
Overview of cytoplasmic and nuclear protein interactions contributing to Ataxin-1 solubility and pathology. SCA1 pathology is associated with the formation insoluble deposits in the nucleus. However, they also occur in brain regions which do not degenerate. The current opinion in the field is that these nuclear inclusions have a protective role, while a soluble form of Ataxin-1 is the major driver of pathology.7 In the cytoplasm, phosphorylated Ataxin-1 (blue, with an ordered AXH domain flanked by disordered N- and C-termini) interacts with 14-3-3 (green). This interaction protects Ataxin-1 against proteasomal degradation13 and aggregation. Upon release from 14-3-3, Ataxin-1 can translocate to the nucleus and – when polyQ expanded – exert its neurodegenerative effect by altering nuclear protein interactions. Capicua (purple) has been shown to stabilise Ataxin-1 by forming soluble oligomeric complexes. The higher abundance of CIC in the cerebellum potentially explains why this region is more vulnerable to neurodegeneration than other regions in the brain.42 Another nuclear interaction that has been shown to contribute to SCA1 pathology is that between polyQ expanded, phosphorylated Ataxin-1 and RBM17 (orange).6