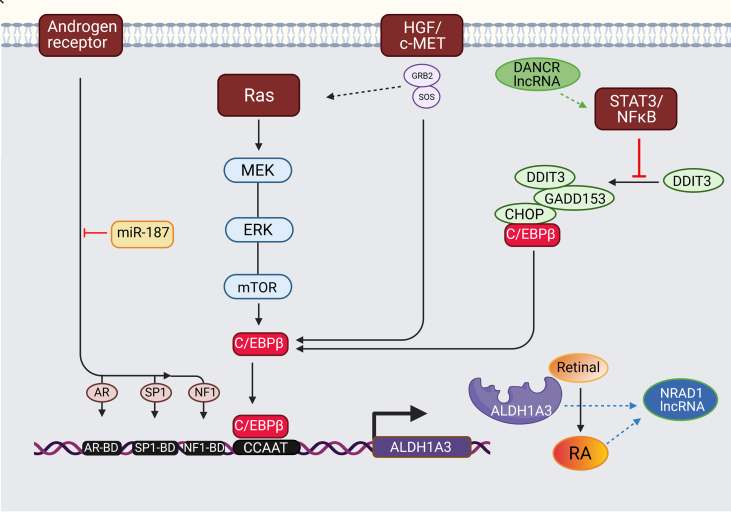
Fig. 3.
Transcriptional regulation of ALDH1A3.
The human ALDH1A3 gene promoter contains a CCAAT box (71 to 67 bp) activated by CCAAT/enhancer-binding protein beta (C/EBPβ). The binding of C/EBPβ to the promoter activates ALDH1A3 expression. ALDH1A3 impacts CSCs through the biosynthesis of retinoic acid (RA).
K-RAS. A key regulator of ALDH1A3 expression is the K-RAS oncogene. Signalling from K-RAS to C/EBPβ2 is likely through phosphorylation and subsequent activation of C/EBPβ2, allowing C/EBPβ2 to activate genes involved in proliferation and EMT transcriptionally. In a pancreatic cancer model using transgenic mice, mutant K-RAS upregulates ALDH1A3 expression via the MEK/ERK/mTOR pathways, a crucial regulator of cell proliferation, differentiation, and apoptosis [49].
STAT3. Aberrant activation of STAT3 and subsequent interactions with apoptosis-related or angiogenic factors can promote tumour progression. Activation of the STAT3 pathway significantly promoted ALDH1A3 expression [50]. Without the activity of STAT3-NFκB, C/EBPβ forms a complex with DDIT3/CHOP/GADD153. STAT3-NFκB activity represses the formation of the complex leading to C/EBPβ-dependent ALDH1A3 promoter activation [51]. The inhibition of STAT3-NFκB activity increases DDIT3 expression and DDIT3-C/EBPβ complex formation, which reduces the occupancy of the ALDH1A3 promoter by C/EBPβ and suppresses ALDH1A3 expression [9], [51].
Androgen Receptor (AR). ALDH1A3 expression is also directly regulated by AR signalling in androgen-dependent prostate cancer (PCa) cell line expressing ALDH1A3 only [52]. The UTR of the ALDH1A3 promoter contains potential DNA binding domains for AR and its transcription factors Sp1 and NF1 located upstream of the transcription initiation site. The addition of androgen DHT to PCa cells significantly increased the expression and the activity of ALDH1A3. At the same time, genetic and pharmacological inhibition of AR eliminated the ability of DHT to induce ALDH1A3 mRNA. DHT upregulated ALDH1A3 directly through the induction of AR transcriptional activity and not via indirect involvement of ERK and Src kinase pathways [52]. A consecutive study identified ALDH1A3 as a miR187 target in prostate cancer. ALDH1A3 expression was inversely correlated to miR-187 and significantly downregulated in PCa cell lines after transient transfection of miR-187 precursor [53].
HGF/c-MET. Genetic or pharmacological inhibition of c-MET in pancreatic cancer cells significantly decreased ALDH1A3 expression and activity. Activation of the HGF/c-MET pathway significantly promoted ALDH1A3 expression. However, the specific mechanism is unknown [54].
Long ncRNA. DANCR impacts ALDH1A3 by derepression of β-catenin, leading to an increase of the stemness traits of hepatocellular carcinoma [67] and elevation of activated TGF-β and IL-6, NFκB and STAT3 production in TNBC [68]. In the same cancer model, DANCR regulates the promoter binding of EZH2 and stimulates the expression of CSC markers, MDR and ALDH1 [64]. The expression of NRAD1 is regulated by ALDH1A3 and its product RA [62].
Abbreviations: AR - Androgen Receptor, C/EBPβ - CCAAT/enhancer binding protein β, CHOP - C/EBP homologous protein, DDIT3 - DNA damage inducible transcript 3, DHT - dihydrotestosterone, ERK - extracellular signal-regulated kinase, EZH2 - enhancer of zeste homolog 2, GADD153 - growth arrest and DNA damage 153, HGF - hepatocyte growth factor, MDR - multidrug resistance, MEK - mitogen-activated protein kinase, mTOR - mammalian target of rapamycin, RA - retinoic acid.