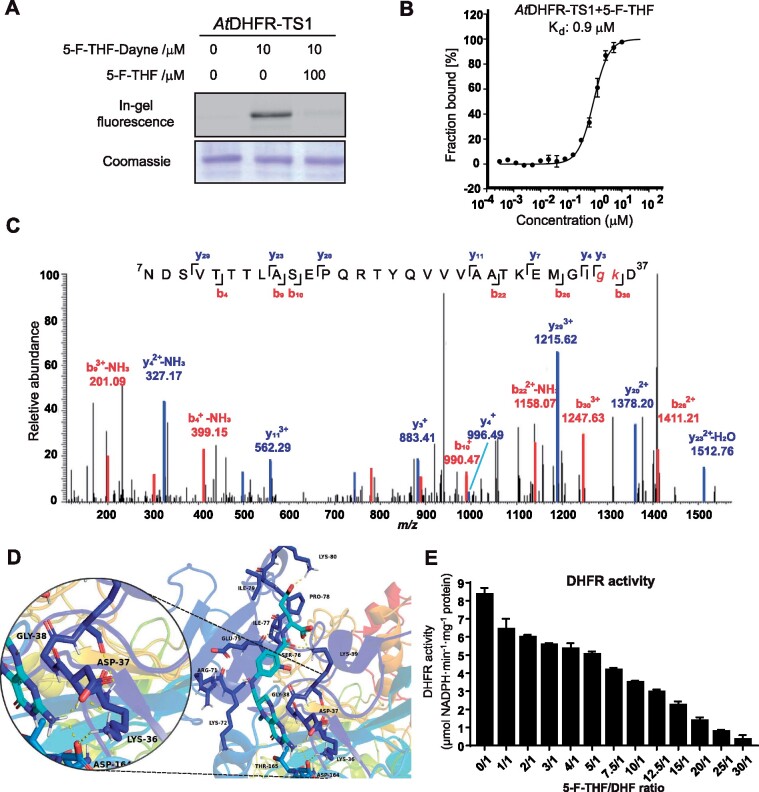
Figure 6.
In vitro characterization of the binding of 5-F-THF with the recombinant Arabidopsis AtDHFR-TS1. A, Gel-based labeling results showing the binding of 5-F-THF-Dayne (10 μM) with AtDHFR-TS1. B, Dose-response curve reflecting binding of the recombinant AtDHFR-TS1 with 5-F-THF as assessed by MST analysis. The curve was generated by NanoTemper Analysis version 1.2.231. Normalized fluorescence (hot fluorescence/initial fluorescence) was plotted as a function of 5-F-THF concentration. Three independent thermophoresis measurements were performed; results are shown as mean � SE of the three repetitions. C and D, Identification of the binding site of 5-F-THF in AtDHFR-TS1. C, LC–MS/MS revealed that G35 or K36 is the potential binding position of 5-F-THF-Dayne in AtDHFR-TS1. G, glycine; K, lysine. D, Molecular docking prediction of the binding of 5-F-THF with AtDHFR-TS1. 5-F-THF is represented in light blue and hydrogen bonds are represented with dotted yellow lines. E, Enzymatic activity of the native AtDHFR-TS1 under conditions of varying 5-F-THF/ DHF ratio (50 �M DHF was used). DHFR activity values are means � se of three independent reactions. For the corresponding enzyme kinetics curve showing NADPH absorbance at 340 nm, see Supplemental Figure S8.