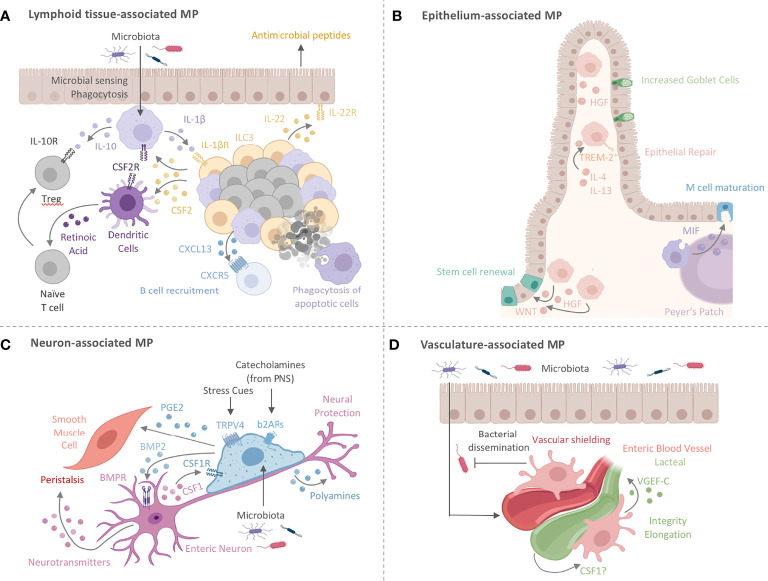
Figure 2.
Intestinal MPs play multiple non-immune roles to maintain tissue homeostasis. (A) Lymphoid tissue -associated MPs (lMPs) accumulate in PPs, ILFs and CPs. These lMPs sense and sample microbes and surround the B cell follicles within PPs and ILFs. Microbial recognition by lMPs facilitates the release of IL-1β and activates lymphoid tissue-resident group 3 innate lymphoid cells (ILC3s). ILC3s release CSF2 and IL-22 to engage CSF2R on myeloid cells or IL-22R on epithelial cells. The latter is prominent in facilitating antimicrobial activity. The former (CSF2-CSF2R) acts on DCs and lMPs to induce the production of IL-10 and RA, both critical in facilitating the conversion of naïve T cells in to regulatory T cells (Treg). Lymphoid tissue -associated MPs have also been shown to release the B cell-recruiting chemokine CXCL13 and clear apoptotic B cells resulting from failed somatic hypermutation or class-switch recombination, thus contributing to the local IgA response. (B) MPs lining the epithelium (eMPs) near intestinal crypts induce stem cell renewal by inducing WNT signaling in intestinal epithelial stem cells (IESC). Epithelium-associated MPs adopt an alternative activation phenotype when stimulated with by IL-4 and IL-13, by upregulating TREM-2 and aiding in epithelial repair and goblet cell proliferation. MPs in the subepithelial dome of the PPs have been shown to induce M cell maturation through the release of MIF. Hepatocyte growth factor (HGF) is another protein that mediates epithelial repair and is possibly produced by eMPs post injury with likely varying locations around the crypt and the villus. (C) Nerve-associated MPs (nMPs) in the intestinal muscularis layer are stimulated by the gut microbiota and regulate peristalsis via BMP2-mediated activation of enteric neurons. Enteric neurons release neurotransmitters to induce smooth muscle cell contractions. Direct activation of smooth muscle cell contraction is mediated by nMP-release of PGE2. In addition, nMPs release polyamines in response to signals from the microbiome, catecholamines, and other stress cues to facilitate neuronal protection. In turn, enteric neurons secrete CSF1 to support nMP survival. (D) Across the lamina propria, vasculature-associated MPs (vMPs) wrap around blood vessels to aid in angiogenesis, lipid transport, dead cell clearance, vessel integrity and elongation. In the presence of an intact microbiota, these MPs are rapidly replaced by monocyte-derived cells to induce vascular repair while protecting against bacterial dissemination upon injury and infection. How the endothelium in turn maintains MP survival is currently unknown. Vasculature-associated MPs in close proximity to small intestinal lacteals facilitate vessel elongation and integrity through the microbiota-driven production of VGEF-C.