Figure 5. Specific Antimigraine Treatment in Dosages With Widespread Clinical Use as Regards to Adverse Events.
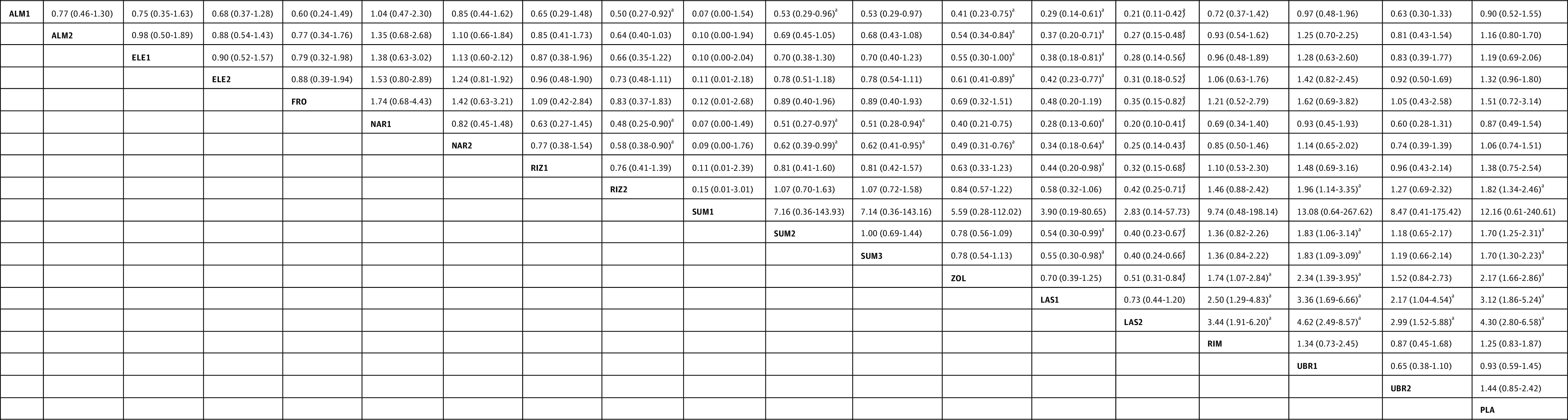
Comparisons between treatment must be read from left to right and the estimates (odds ratio with 95% CI) lower than 1 favor column-defining treatment. ALM1 indicates almotriptan, 6.25 mg; ALM2, almotriptan, 12.5 mg; ELE1, eletriptan, 20 mg; ELE2, eletriptan, 40 mg; FRO, frovatriptan, 2.5 mg; LAS1, lasmiditan, 50 mg; LAS2, lasmiditan, 100 mg; NAR1, naratriptan, 1 mg; NAR2, naratriptan, 2.5 mg; PLA, placebo; RIM, rimegepant, 75 mg; RIZ1, rizatriptan, 5 mg; RIZ2, rizatriptan, 10 mg; SUM1, sumatriptan, 10 mg nasal spray; SUM2, sumatriptan, 50 mg; SUM3, sumatriptan, 100 mg; UBR1, ubrogegant, 50 mg; UBR2, ubrogepant, 100 mg; and ZOL, zolmitriptan, 2.5 mg.
aP < .05.
