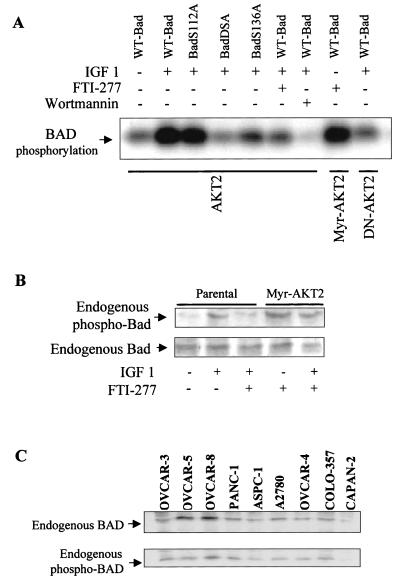
FIG. 5.
AKT2 phosphorylates recombinant BAD and BADS112A but not BADS136A or BAD2SA: inhibition by FTI-277 in vitro and in vivo. (A) In vitro kinase assays using anti-HA immunoprecipitates from COS7 cells transfected transiently with HA-tagged AKT2 constructs expressing wild-type AKT2 (HA-AKT2), constitutively active AKT2 (Myr-AKT2), and dominant-negative mutant AKT2 (AKT2-E299K). Following serum starvation, the cells were treated with or without FTI-277 (3 h) or wortmannin (30 min) prior to IGF-1 (50 ng/ml) stimulation. Anti-HA immunoprecipitates were subjected to an in vitro kinase assay using wild-type BAD (WT-Bad), BADS112A, BADS136A, or BAD2SA as the substrate. Note that wortmannin inhibited BAD phosphorylation more efficiently than FTI-277 due to relatively short time of treatment of the cells with FTI-277 (3 h; see Fig. 8). (B) Western blot analysis of phosphorylation (top) and expression (bottom) of endogenous BAD from parental OVCAR-3 cells and the stably transfected cell clones expressing Myr-AKT2 treated with or without FTI-277 before stimulation with IGF-1 (50 ng/ml) for 10 min. (C) Western blot analyses of cell lysates from ovarian and pancreatic cancer cell lines. The blots were detected by anti-BAD (top) or anti-p-BAD (bottom) antibody.