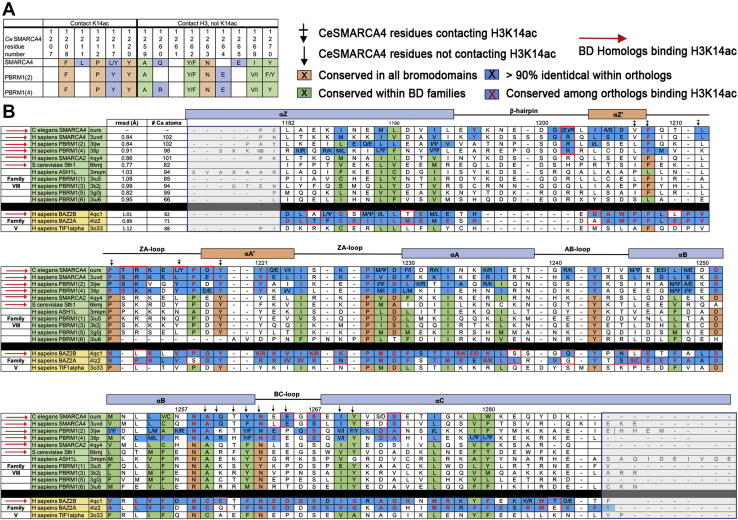
Figure 4.
Conserved residues in bromodomains that recognize H3K14ac.A, based on sequence conservation, the 15 residues in direct contact with the H3 peptide do not determine sequence specificity. Only the conserved residues are listed here—blank boxes are not conserved. The four orange boxes are conserved in all BDs, and the four green boxes are conserved within BD families (shaded as in (1)). Boxes colored blue are conserved for that paralog (SMARCA4 or PBRM1(2) or PBRM1(4)). The sidechains of Glu1264 or Glu1265 contact H3R17 and are the only paralog-specific residues that contact a sidechain of H3 outside of the K14ac binding pocket. B, the CeSMARCA4 BD sequence is aligned with other H sapiens family VIII and family V sequences. The 15 residues contacting the H3K14ac peptide in CeSMARCA4 are denoted as black arrows. Boxes are colored as in A. Boxes with red outlines identify conserved residues shared in family VIII BDs that bind the H3K14ac mark or residues that distinguish BAZ2A and BAZ2B BD orthologs. Secondary structure elements are marked along the top. Numbers above the sequence refer to the CeSMARCA4 sequence. The alignment is based on structures (pdbid, third column) superimposed with the CeSMARCA4 structure resulting in an overall root mean square deviation (rmsd, fourth column) of residues less than 2 Å apart after superposition (number of Cα atoms, fifth column). Structures with longer sequences outside of the aligned regions are included in the grey boxes at the N- and C-termini of the alignment. Ψ, hydrophobic residue; A1/A2, two similar residues occurring in the alignment.