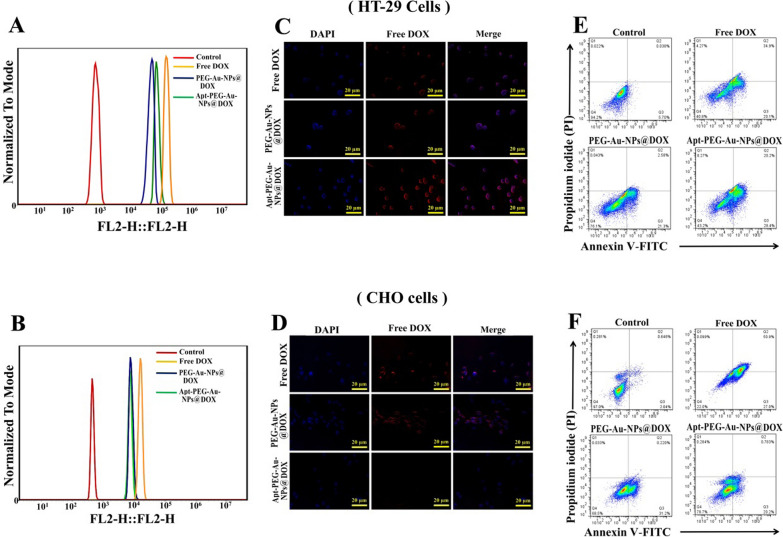
Fig. 5.
Cellular internalization and cell death mechanism analysis. The cellular uptake of free DOX and nanocarriers was investigated by both flow cytometry and fluorescence microscopy. HT-29 and CHO cells were treated with free DOX, PEG-Au-NPs@DOX and Apt-PEG-Au-NPs@DOX (DOX final concentration was 5 μg/ml) for 6 h at 37 °C. Flow cytometry histograms of A HT-29 and B CHO cells after treatment with free DOX, PEG-Au-NPs@DOX and Apt-PEG-Au-NPs@DOX. The results of cell internalization of free DOX and nanocarriers on C HT-29 and D CHO cells as visualized by fluorescent microscopy. Nuclei were stained with DAPI. Scale bar = 20 μm. Cell death mechanism was assessed by Annexin V-FITC/PI staining using flow cytometry. The results showed that free DOX, PEG-Au-NPs@DOX and Apt-PEG-Au-NPs@DOX induced apoptotic pathway in E HT-29 and F CHO cells. Viable, early, and late apoptotic cell populations accumulated in Q4, Q3, and Q2, respectively. DOX Doxorubicin, SPION Superparamagnetic iron oxide nanoparticle, MSN Mesoporous silica nanoparticle, PEG Polyethylene glycol, NP nanoparticle, Apt Aptamer, EpCAM Epithelial cell adhesion molecule, HT-29 cells Human colorectal adenocarcinoma cells, CHO cells Chinese hamster ovary cells