Abstract
Background
Post-tuberculosis bronchomalacia (PTBM) is one of the main conditions occurring in patients after tracheobronchial tuberculosis (TBTB), and is also associated with the recurrence of symptoms. The present study aimed to investigate the predictors of PTBM in patients who had been undergoing appropriate TB treatment.
Material/Methods
Clinical data of 104 patients with symptomatic airway stenosis after TBTB between January 01, 2019 and June 31, 2020 were recorded and analyzed. The association between baseline clinical characteristics, laboratory results, and PTBM was calculated with logistical regression. The time from onset of bronchoscopic intervention was examined by Kaplan-Meier estimates; differences between the 2 groups were tested by the log-rank test.
Results
Fifty-seven patients (54.81%) had PTBM. In the multivariate logistical analysis, the left main bronchus stenosis lesion (odds ratio [OR]=3.763), neutrophil (NEUT) count (OR=1.527), and platelet (PLT) (OR=1.010) count were predictors of PTBM. During follow-up, patients with BM had a significantly longer duration from onset of bronchoscopic intervention than patients without BM (hazard ratio=2.412, P<0.0001). Further, all patients needing long-term bronchoscopic intervention therapy were subsequently identified as having PTBM. Additionally, blood PLT counts were significantly decreased to normal levels in the non-BM group (P<0.05), but not in the BM group (P>0.05).
Conclusions
PTBM is most likely to occur in the left main bronchus. The inflammatory and immune responses associated with NEUT and PLT may represent therapeutic targets of PTBM. Our study is the first to report that decreased blood PLT count has the potential to monitor the treatment response.
Keywords: Blood Platelets, Neutrophils, Tracheobronchomalacia
Background
Tracheomalacia is characterized by weakening of the airway wall and dynamic collapse of the airway lumen during respiration [1–4]; the condition is called tracheobronchomalacia if the main bronchi are also affected [5]. At present, there is no generally accepted adult classification of the causes of tracheomalacia. Tracheomalacia may result from tumor invasion [6], cicatricial tracheal stenosis associated with a tracheal tube [7], trauma and surgery [8], compression of an abnormal artery [9,10], or goiter [11], and can be associated with esophageal achalasia [12], cystic fibrosis [13], and chronic obstructive pulmonary disease [14].
Tracheobronchial tuberculosis (TBTB) is a tuberculosis (TB) infection of the trachea and bronchus, and has been reported in 10–50% of patients with pulmonary TB (PTB) [15,16]. Due to the residual and long-term damage after TBTB, 90% of patients with TBTB develop some degree of airway stenosis in the long term, despite adequate antituberculous therapy [17,18]. Post-tuberculosis bronchomalacia (PTBM) is one of the main conditions affecting patients after TBTB [15,19,20]. Consistent with our clinical observations, Lee et al [15] reported that PTBM is associated with the recurrence of symptoms after TBTB. Therefore, in addition to antituberculous treatment, monitoring and determining PTBM is important for early diagnosis and treatment. However, no studies to date have investigated the predictors associated with PTBM. Therefore, we performed this retrospective study to investigate the predictors of PTBM in patients who had been undergoing appropriate TB treatment.
Material and Methods
2 Study Design and Population
We analyzed the medical records of 104 consecutive hospitalized patients with symptomatic airway stenosis after TBTB in the Department of Respiratory and Critical Care Medicine at the First Affiliated Hospital of Chongqing Medical University between January 01, 2019 and June 31, 2020. All these patients had been undergoing appropriate TB treatment for at least 6 months. The exclusion criteria were active TB, malignant and other infectious diseases, and evidence of other systemic diseases. Data regarding clinical characteristics, laboratory examination, chest computed tomography (CT), bronchoscopic intervention treatment, and outcome were obtained from the patients’ medical records. The final date of follow-up was December 31, 2020. The study was conducted in accordance with the Declaration of Helsinki and Uniform Requirements for Manuscripts Submitted to Biomedical Journals, and the protocol was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (Approval ID: No 2020-147).
Laboratory Examination Variables
The laboratory examination included complete blood counts and liver-kidney function. A complete blood count included white blood cell (WBC), red blood cell (RBC), neutrophil (NEUT), lymphocyte (LYM), and platelet (PLT) counts, and hemoglobin (Hb) levels. Liver-kidney function tests included total protein (TP), albumin (Alb), total bilirubin (Tbil), alanine transaminase (ALT), aspartate aminotransferase (AST), glutamyl transpeptidase (GGT), and creatinine (Crea) levels.
Diagnostic Criteria for PTBM and Bronchoscopic Intervention
PTBM was diagnosed according to Chung’s classification [20] using a bronchoscope (CV-290; Olympus, Tokyo, Japan). All symptomatic patients with airway stenosis after TBTB were treated using bronchoscopic intervention according to treatment guidelines [21] using an electrocautery needle knife (VIO 300S, ERBE, Tubingen, Germany), a dilatation balloon (ENDO-FLEX, Voerde, Germany), and a multi-use cryosurgery system (ERBOKRYO CA, ERBE, Tuebingen, Germany). Patients were followed up for at least 6 months after the last intervention. As the high incidence of recurrence is the most common problem among patients with PTBM, we therefore investigated the duration of bronchoscopic intervention after the onset of intervention in this population.
Statistical Analysis
Normality was tested using the Shapiro-Wilk test to separate parametric from non-parametric variables. The continuous variables of normally distributed data are expressed as means±standard deviation and differences between any 2 groups were evaluated using the unpaired t test. Non-normally distributed data are expressed as median (interquartile range) and the differences were evaluated using the Mann-Whitney U test. Changes between 2 related groups were tested using the matched-pair t test for normally distributed variables and the Wilcoxon matched-pair signed-rank test for non-normally distributed variables. Categorical data are expressed as numbers (percentage), and comparisons for testing statistically significant differences were made using the χ2 test (minimum expected values ≥5) or Fisher’s exact test (minimum expected values <5). The association between baseline clinical characteristics, laboratory results, and PTBM was calculated with logistic regression analysis. The time from onset of bronchoscopic intervention was examined by Kaplan-Meier estimates, and differences between the 2 groups were tested by the log-rank test. Statistical analyses were performed using SPSS version 20.0 (SPSS Software, Inc., Chicago, IL, USA), and P<0.05 was considered statistically significant (all P values are from 2-sided tests). Graphical analysis was performed using GraphPad Prism 6.05 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Diagnosis and Incidence of PTBM
There were 104 consecutive hospitalized patients with airway stenosis after TBTB who underwent bronchoscopy; all the patients had cicatrices strictures, and 57 (54.81%) had PTBM, but other types of stenosis were not found. The chest HRCT and bronchoscopy features of patients with PTBM are shown in Figure 1. All patients underwent laboratory examination, chest CT, and bronchoscopic intervention on primary admission.
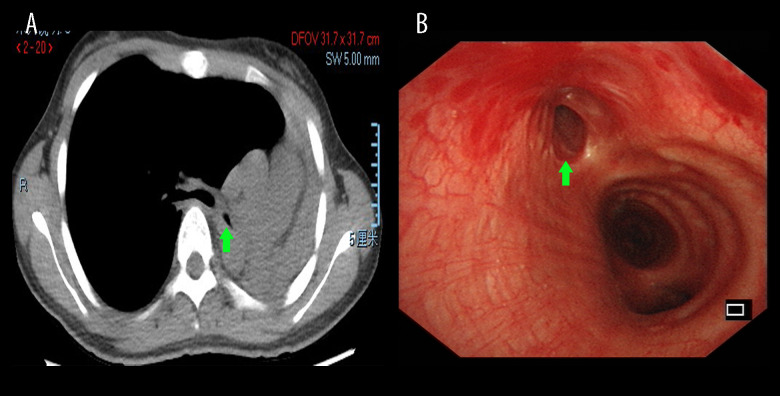
Figure 1. Chest HRCT and bronchoscopy features of patients with PTBM.
Chest HRCT showing left main bronchus stenosis lesion (A, green arrow) due to TBTB. Diagnostic bronchoscopic appearance of PTBM (B, green arrow) can be observed in the left main bronchus. HRCT – high-resolution computed tomography; PTBM – post-tuberculosis bronchomalacia; TBTB – tracheobronchial tuberculosis.
Clinical Characteristics of the Study Participants
The relevant clinical characteristics of patients with BM and non-BM are summarized in Table 1. The significant clinical characteristics included age, sex, residence, smoking, bronchoscopic intervention history, and symptoms at presentation. Compared to patients without BM, those with BM were more likely to live in a rural area, and had a shorter duration from symptom onset to intervention, more acute intervention therapy, more stent placement, and longer average intervention interval history (all, P<0.05; Table 1).
Table 1.
Clinical characteristics of study participants.
| Characteristics | BM (n=57) | Non-BM (n=47) | Z/χ2 | P-value |
|---|---|---|---|---|
| Age, years | 29.0 [22.5–35.0] | 31.0 [24.0–43.0] | −1.223 | 0.221a |
| <40 | 45 (78.95%) | 33 (70.21%) | 1.048 | 0.306b |
| ≥40 | 12 (21.05%) | 14 (29.79%) | ||
| Sex, n (%) | NA | 0.337c | ||
| Male | 8 (14.04%) | 3 (6.38%) | ||
| Female | 49 (85.96%) | 44 (93.62%) | ||
| Residence, n (%) | 5.684 | 0.017b | ||
| Urban | 15 (26.32%) | 23 (48.94%) | ||
| Rural | 42 (73.68%) | 24 (51.06%) | ||
| Smoking, n (%) | NA | 1.000c | ||
| Yes | 3 (5.26%) | 2 (4.26%) | ||
| No | 54 (94.74%) | 45 (95.74%) | ||
| Duration from PTB to TBTB diagnosis, months, n (%) | 1.390 | 0.238b | ||
| <4 | 18 (31.58%) | 10 (21.28%) | ||
| ≥4 | 39 (68.42%) | 37 (78.72%) | ||
| Duration from symptom onset to intervention, months | 7.507 | 0.006b | ||
| <1 | 36 (63.16%) | 17 (36.17%) | ||
| ≥1 | 21 (36.84%) | 30 (63.83%) | ||
| Acute intervention history | NA | 0.031c | ||
| Yes | 6 (10.53%) | 0 (0.00%) | ||
| No | 51 (89.47%) | 47 (100.00%) | ||
| Stent placement history | 7.441 | 0.006b | ||
| Yes | 11 (19.30%) | 1 (2.13%) | ||
| No | 46 (80.70%) | 46 (97.87%) | ||
| Average intervention interval, months | 23.662 | <0.001b | ||
| <3 | 43 (75.44%) | 13 (27.66%) | ||
| ≥3 | 14 (24.56%) | 34 (72.34%) | ||
| Symptoms at presentation, n (%) | ||||
| Cough | 0.053 | 0.818b | ||
| Yes | 40 (70.18%) | 32 (68.09%) | ||
| No | 17 (29.82%) | 15 (31.91%) | ||
| Dyspnoea | 1.646 | 0.200b | ||
| Yes | 29 (50.88) | 18 (38.30%) | ||
| No | 28 (49.12%) | 29 (61.70%) | ||
| Wheeze | 3.850 | 0.050b | ||
| Yes | 13 (22.81%) | 4 (8.51%) | ||
| No | 44 (77.19%) | 43 (91.49%) |
The data are expressed as median (interquartile range) or numbers (percentage). Differences between the 2 groups were tested by corresponding statistical tests. The details are as follow:
the data were analyzed by Mann-Whitney U test for non-normally distributed continuous variables;
the data were analyzed by the χ2 test (minimum expected values ≥5);
the data were analyzed by Fisher’s exact test (minimum expected values <5).
BM – bronchomalacia; PTB – pulmonary tuberculosis; TBTB – tracheobronchial tuberculosis; NA – not available.
There were no significant differences in age, sex, smoking, duration of PTB to TBTB diagnosis, and cough, dyspnea, or, more wheeze symptoms at presentation between patients with BM and those without BM (P>0.05).
Chest CT and Bronchoscopy Features of Study Participants
The chest CT and bronchoscopy features are summarized in Table 2. The chest CT features included atelectasis, cavitary, fibrous lesion, mucus plugging, and bronchiectasis. There were no significant differences in any features between patients with BM and those without BM (P>0.05).
Table 2.
Chest CT and bronchoscopy features of study participants.
| Characteristics | BM (n=57) | Non-BM (n=47) | χ2 | P-value |
|---|---|---|---|---|
| Chest CT features, n (%) | ||||
| Atelectasis | 0.878 | 0.349 | ||
| Yes | 14 (24.56%) | 8 (17.02%) | ||
| No | 43 (75.44%) | 39 (82.98%) | ||
| Cavitary | 0.127 | 0.722 | ||
| Yes | 6 (10.53%) | 6 (12.77%) | ||
| No | 51 (89.47%) | 41 (87.23%) | ||
| Fibrous lesion | 0.016 | 0.900 | ||
| Yes | 9 (15.79%) | 7 (14.89%) | ||
| No | 48 (84.21%) | 40 (85.11%) | ||
| Mucus plugging | 2.015 | 0.156 | ||
| Yes | 15 (20.07%) | 7 (14.89%) | ||
| No | 42 (71.93%) | 40 (85.11%) | ||
| Bronchiectasis | 0.036 | 0.849 | ||
| Yes | 22 (38.60%) | 19 (40.43%) | ||
| No | 35 (61.40%) | 28 (59.57%) | ||
| Bronchoscopic features, site, n (%) | ||||
| Trachea | 4.352 | 0.037 | ||
| Yes | 17 (29.82%) | 6 (12.77%) | ||
| No | 40 (70.18%) | 41 (87.23%) | ||
| Left main bronchus | 7.717 | 0.005 | ||
| Yes | 35 (61.40%) | 16 (34.04%) | ||
| No | 22 (38.60%) | 31 (65.96%) | ||
| Right main bronchus | 0.540 | 0.463 | ||
| Yes | 17 (29.82%) | 11 (23.40%) | ||
| No | 40 (70.18%) | 36 (76.60%) | ||
| Bronchus intermedius | 0.272 | 0.602 | ||
| Yes | 8 (14.04%) | 5 (10.64%) | ||
| No | 49 (85.96%) | 42 (89.36%) | ||
| ≥2 bronchi | 5.136 | 0.023 | ||
| Yes | 18 (31.58%) | 6 (12.77%) | ||
| No | 39 (68.42%) | 41 (87.23%) | ||
| Stenosis degree | 2.899 | 0.089 | ||
| Mild-to-moderate | 22 (38.60%) | 26 (55.32%) | ||
| Severe | 35 (61.40%) | 21 (44.68%) | ||
Data are expressed as numbers (percentage). Differences between the 2 groups were tested by the χ2 test for categorical variables. BM – bronchomalacia; CT – computed tomography.
The bronchoscopy features primarily included stenosis lesion sites and stenosis degree. Compared to patients without BM, patients with BM had more trachea or left main bronchus stenosis lesions, and had ≥2 bronchi stenosis lesions more frequently (P<0.05). The differences did not vary significantly in the right main bronchus and bronchus intermedius stenosis lesions (P>0.05). Although significant differences were not found, there was a trend for patients with BM to have more severe airway stenosis than mild-to-moderate airway stenosis (χ2=2.908, P=0.088).
Laboratory Examination of Study Participants
The results of the laboratory examinations are summarized in Table 3. Compared to patients without BM, those with BM had higher blood WBC, NEUT, and PLT counts; as well as a higher NEUT/LYM ratio (all, P<0.05). NEUT and PLT counts remained elevated in the initial stage. After bronchoscopic intervention, symptoms improved immediately, and about 2 weeks later NEUT and PLT counts began to fall in all the enrolled patients. Then, NEUT and PLT counts continued to increase as airway stenosis and bronchomalacia (BM) worsened. No significant differences were found in blood RBC counts, Hb levels, and LYM counts between the 2 groups (P>0.05). Regarding liver-kidney function, the blood TP, Alb, Tbil, ALT, AST, GGT, and Crea levels were not significantly different between BM and non-BM patients (P>0.05).
Table 3.
Laboratory examination of the study participants.
| Variables | BM (n=57) | Non-BM (n=47) | t/Z | P-value |
|---|---|---|---|---|
| Complete blood counts | ||||
| WBC (×109/L) | 5.89 [4.74–8.14] | 4.97 [4.02–5.96] | −3.361 | 0.001 |
| RBC (×1012/L) | 4.36 [4.15–4.61] | 4.47 [4.26–4.79] | −1.016 | 0.310 |
| Hb (g/L) | 132.07±14.80 | 129.70±13.47 | 0.853 | 0.396 |
| NEUT (×109/L) | 4.22 [2.89–6.15] | 3.33 [2.28–3.99] | −3.406 | 0.001 |
| LYM (×109/L) | 1.28 [1.01–1.80] | 1.28 [0.98–1.50] | −0.892 | 0.373 |
| NEUT/LYM ratio | 2.89 [2.10–4.32] | 2.45 [1.80–3.22] | −2.364 | 0.018 |
| PLT (×109/L) | 262.00 [209.00–310.00] | 217.00 [179.00–248.00] | −3.070 | 0.002 |
| Liver-kidney function | ||||
| TP (g/L) | 73.67±6.36 | 73.94±6.17 | −0.121 | 0.904 |
| Alb (g/L) | 42.79±3.62 | 42.89±3.25 | −0.046 | 0.963 |
| Tbil (μmol/L) | 9.00 [5.65–15.20] | 8.40 [5.90–9.80] | −0.849 | 0.396 |
| ALT (U/L) | 20.00 [15.50–25.00] | 18.00 [13.00–24.00] | −1.554 | 0.120 |
| AST (U/L) | 21.00 [18.00–24.00] | 20.00 [16.00–25.00] | −0.170 | 0.865 |
| GGT (U/L) | 21.00 [14.00–32.50] | 16.00 [13.00–25.00] | −1.423 | 0.155 |
| Crea (μmol/L) | 51.00 [47.00–59.00] | 47.00 [44.00–54.00] | −1.939 | 0.053 |
Data are expressed as means±standard or median (interquartile range). Differences between the 2 groups were tested by the unpaired t test for normally distributed continuous variables and the Mann-Whitney U test for non-normally distributed continuous variables. BM – tracheomalacia; WBC – white blood cell; RBC – red blood cell; NEUT – neutrophil; LYM – lymphocyte; Hb – hemoglobin; PLT – platelet; TP – total protein; Alb – albumin; Tbil – total bilirubin; ALT – alanine transaminase; AST – aspartate aminotransferase; GGT – glutamyl transpeptidase; Crea – creatinine.
Predictors Associated with PTBM
Among the variables that showed significant differences in the univariate analysis (Tables 1–3), 4 were selected using the forward stepwise method for the multivariate logistic regression model for PTBM. In the univariate analysis, living in a rural area, left main bronchus stenosis lesion, NEUT count, and PLT count were all associated with the development of PTBM (Table 4). In the multivariate logistic analysis, left main bronchus stenosis lesion, NEUT count, and PLT counts were independently associated with PTBM. Living in a rural area was excluded, which suggested that living in a rural area was not independently related to PTBM (Table 4).
Table 4.
Predictors of PTBM.
| Univariate logistic regression | Multivariate logistic regression | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Rural, n (%) | 2.683 | 1.180–6.101 | 0.019 | 1.774 | 0.680–4.625 | 0.241 |
| Left main bronchus, n (%) | 2.187 | 0.145–0.726 | 0.009 | 3.763 | 1.478–9.579 | 0.005 |
| NEUT (×109/L) | 1.700 | 1.246–2.319 | 0.001 | 1.527 | 1.079–2.160 | 0.017 |
| PLT (×109/L) | 1.010 | 1.003–1.017 | 0.004 | 1.010 | 1.002–1.018 | 0.013 |
The association between baseline clinical characteristics, laboratory results, and TPBM was calculated with logistic regression analysis. NEUT – neutrophil; PLT – platelet; OR – odds ratio; CI – Confidential interval.
Outcomes of the Study Participants
Symptoms improved immediately after bronchoscopic intervention in all the enrolled patients. As shown in Figure 2, the time from onset of bronchoscopic intervention was significantly longer in patients with BM than that in patients without BM (hazard ratio [HR]=2.412, P<0.0001). During follow-up, there were 6 (10.53%) patients in the BM group and 2 (4.26%) patients in the non-BM group who required long-term bronchoscopic intervention therapy to restore airway patency. Further, the patients needing long-term bronchoscopic intervention therapy in the BM and non-BM groups were all subsequently identified as having BM.
Figure 2. Duration of bronchoscopic intervention therapy in BM and non-BM groups.
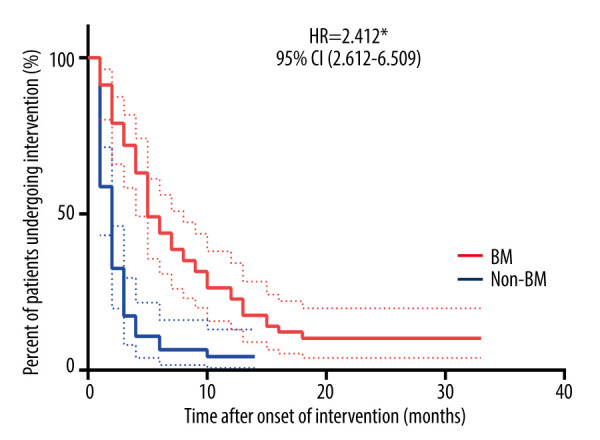
The time from onset of bronchoscopic intervention was examined by Kaplan-Meier estimates. Differences between the 2 groups were tested by the log-rank test. * P<0.0001. BM – bronchomalacia; HR – hazard ratio; CI – confidential interval.
The complete blood count results from 84 (53 with BM, 31 with non-BM) patients were used to study the long-term effect of bronchoscopic intervention therapy. The changes are summarized in Table 5. PLT counts were significantly decreased to normal levels in the non-BM group (P<0.05), but not in the BM group (P>0.05). The PLT counts in both groups showed a fluctuating trend in the long-term follow-up. The PLT counts in the non-BM group gradually decreased to normal levels, while the PLT counts in the BM group were always higher than normal levels. Regarding WBC, RBC, Hb, NEUT, and LYM levels, the changes were not significant in both groups (P>0.05).
Table 5.
Comparison of long-term changes of complete blood counts between the 2 groups.
| Variables | BM | Non-BM | ||||
|---|---|---|---|---|---|---|
| N=53 | Z | P-value | N=31 | Z | P-value | |
| Complete blood counts | ||||||
| WBC (×109/L) | 0.09 [−2.16–1.43] | −0.806 | 0.420 | 0.19 [−0.73–1.00] | −0.588 | 0.577 |
| RBC (×1012/L) | 0.10 [−2.21–0.29] | −0.881 | 0.378 | −0.08 [−0.29–0.14] | −1.203 | 0.229 |
| Hb (g/L) | 4.00 [−7.00–13.00] | 1.139 | 0.260 | 1.00 [−4.00–10.00] | 0.900 | 0.375 |
| NEUT (×109/L) | 0.00 [−1.81–0.89] | −1.088 | 0.276 | −0.02 [−0.46–0.46] | −0.216 | 0.829 |
| LYM (×109/L) | 0.15 [−0.25–0.41] | −1.311 | 0.190 | 0.10 [−0.24–0.33] | −0.941 | 0.347 |
| PLT (×109/L) | −16.00 [−73.50–28.50] | −1.651 | 0.099 | −18.00 [−42.00–2.00] | −2.412 | 0.016 |
Data ae expressed as median (interquartile range). Differences between the 2 groups were tested by the Mann-Whitney U test for non-normally distributed continuous variables. BM – bronchomalacia; WBC – white blood cell; RBC – red blood cell; NEUT – neutrophil; LYM – lymphocyte; Hb – hemoglobin; PLT – platelet.
Discussion
This is the first report to date that describes left main bronchus stenosis lesion, NEUT, and PLT counts to be independently associated with PTBM. Epidemiological surveys have suggested that urban residents have higher rates of TBTB in southern China [22]. Supplementing that finding, this study found that rural residents had higher rates of PTBM. This finding might be related to the fact that rural residents had lower quality of life, lower level of education, and poorer compliance with standardized anti-TB treatments than urban residents [23–25]. In the present study, patients with BM were more likely to present with wheeze symptoms than those without BM. Furthermore, patients with trachea or left main bronchus lesions, more than 2 bronchi lesions, and severe airway stenosis had higher rates of BM. These differences may be due to the abnormal tracheobronchial mucosa and cartilage, which impair excretion of sputum to the trachea or bronchi, and gas exchange to the lungs. In addition, the reason why the left main bronchus has a higher probability of BM than the right main bronchus may be related to the diameter and angle of the bronchus, and the pressure of the cardiovascular system in front of the left main bronchus. Consistent with the above results, we also found that patients with BM needed earlier, more urgent, and longer bronchoscopic intervention therapy. Collectively, these results suggest that BM affects the quality of life of patients and is related to worse responses and prognosis of treatment.
As well as targeting the TB bacilli, neutrophils may also trigger hyper-inflammatory [26–28] and innate immune responses [29–31] that lead to tissue damage. The exact role of neutrophils after TBTB remains unclear. Our study showed that patients with BM had significantly higher blood NEUT counts than those without BM. As blood neutrophil counts could reflect the local tracheobronchial status, we suppose that neutrophils may also be related to the occurrence and development of PTBM. Previous studies of PLT have reported an important role for PLT in hemostasis [32]. In addition, the higher blood PLT counts may also indicate increased host inflammatory and immune responses [33–38]. Our results revealed that patients with BM had higher blood PLT counts; thus, the persistent inflammatory and immune responses linked to increased PLT may be related to PTBM. Another credible explanation for the link between the higher PLT counts and a high risk of PTBM is that PLT contributes to plasma levels of TGF-β1 [39,40], which has been proposed as a potential modulator of fibrogenic events and pathways [41–43]. Thus, BM may also be induced by the cicatricial strictures after TBTB. Interestingly, during long-term follow-up, our study showed that PLT counts were fluctuating in both BM and non-BM groups. The PLT counts in the non-BM group gradually decreased to normal levels, while the PLT counts in the BM group were always above normal levels. One possible explanation for this is that bronchoscopic intervention significantly improved the airway stenosis degree, making the decrease of PLT in the non-BM group more obvious. In addition, the higher recurrence rate may be one reason for higher PLT counts in PTBM.
In the present study, patients with BM had a significantly longer average intervention interval than those without BM. Further, the patients needing long-term bronchoscopic intervention therapy in both the BM and non-BM groups were all subsequently identified as having BM. In summary, we have demonstrated that left main bronchus stenosis lesion, NEUT count, and PLT count are independently associated with PTBM. Bronchoscopic intervention is thus far the main strategy for evaluating and restoring the airway patency in airway stenosis after TBTB [44]. However, patients always require regular bronchoscopy follow-up owing to repeated recurrence of airway stenosis symptoms, and may even experience complications from the procedure [15,21,45]. We also showed that BM may be related to bronchoscopic intervention, as 2 patients in the non-BM group were subsequently identified as having BM. Thus, efforts toward reducing nonessential procedures are required, such as reducing NEUT- and PLT-related inflammation and immune responses.
Limitations of the Study
The main limitation of our study is that it was a single-center retrospective study with a relatively small sample size; therefore, the possibility of bias exists. In addition, longer follow-up periods after bronchoscopic intervention are required to evaluate the recurrence of airway stenosis after TBTB. Prospective, multicenter, large-sample studies are needed to confirm our findings.
Conclusions
PTBM is most likely to occur in the left main bronchus. The inflammatory and immune responses associated with NEUT and PLT counts may represent therapeutic targets of PTBM in the future. Non-invasive strategies for monitoring PTBM are clinically important but lacking. Our study is the first to report that decreased blood PLT count has the potential to monitor the treatment response. In future studies, we will enroll more patients in a multicenter study to provide enough data to confirm the findings of the present study.
Acknowledgements
We thank the patients who participated in the study.
Footnotes
Conflict of Interest
None declared.
Declaration of Figures Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: This study was supported by the National Major Science and Technology Projects of China (grant number 2018ZX10302302003), the Natural Science Foundation of Yongchuan District, Chongqing, China (grant number Ycstc, 2020nb0257), and the Funds of Yongchuan Hospital of Chongqing Medical University, Chongqing, China (grant number YJLCX201542)
References
- 1.Hysinger EB, Bates AJ, Higano NS, et al. Ultrashort echo-time MRI for the assessment of tracheomalacia in neonates. Chest. 2020;157:595–602. doi: 10.1016/j.chest.2019.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gunatilaka CC, Higano NS, Hysinger EB, et al. Increased work of breathing due to tracheomalacia in neonates. Ann Am Thorac Soc. 2020;17:1247–56. doi: 10.1513/AnnalsATS.202002-162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hysinger EB, Hart CK, Burg G, et al. Differences in flexible and rigid bronchoscopy for assessment of tracheomalacia. Laryngoscope. 2020;131:201–4. doi: 10.1002/lary.28656. [DOI] [PubMed] [Google Scholar]
- 4.Dewberry L, Wine T, Prager J, et al. Thoracoscopic posterior tracheopexy is a feasible and effective treatment for tracheomalacia. J Laparoendosc Adv Surg Tech A. 2019;29:1228–31. doi: 10.1089/lap.2019.0156. [DOI] [PubMed] [Google Scholar]
- 5.Wallis C, Alexopoulou E, Antón-Pacheco JL, et al. ERS statement on tracheomalacia and bronchomalacia in children. Eur Respir J. 2019;54:1900382. doi: 10.1183/13993003.00382-2019. [DOI] [PubMed] [Google Scholar]
- 6.Xu S, Zhu J, Zhao G, Li S. Tracheal suspension with autogenous rib cartilage in a patient with severe tracheomalacia. J Cardiothorac Surg. 2019;14:21. doi: 10.1186/s13019-019-0840-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brewis C, Pracy JP, Albert DM. Localized tracheomalacia as a complication of the Cole tracheal tube. Paediatr Anaesth. 1999;9:531–33. doi: 10.1046/j.1460-9592.1999.00408.x. [DOI] [PubMed] [Google Scholar]
- 8.Parshin VD, Koroleva IM, Mishchenko MA, et al. [Diagnosis and treatment of acquired tracheomalacia in patients with cicatricial tracheal stenosis]. Khirurgiia (Mosk) 2016;(8):73–82. doi: 10.17116/hirurgia2016873-82. [in Russian] [DOI] [PubMed] [Google Scholar]
- 9.Keng LT, Chang CJ. All that wheezes is not asthma: Adult tracheomalacia resulting from innominate artery compression. Postgrad Med J. 2017;93:54–55. doi: 10.1136/postgradmedj-2016-134177. [DOI] [PubMed] [Google Scholar]
- 10.Kamran A, Friedman KG, Jennings RW, et al. Aortic uncrossing and tracheobronchopexy corrects tracheal compression and tracheobronchomalacia associated with circumflex aortic arch. J Thorac Cardiovasc Surg. 2020;160:796–804. doi: 10.1016/j.jtcvs.2020.03.158. [DOI] [PubMed] [Google Scholar]
- 11.Paul M, Kannaujia A, Chatterjee A, et al. Serial fiber optic bronchoscopy (FOB) to predict the need of tracheostomy in tracheomalacia after thyroidectomy in long standing goiter. J Clin Anesth. 2018;47:9–10. doi: 10.1016/j.jclinane.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 12.De Pieri C, Cogo P, Barbato A. Tracheomalacia due to esophageal achalasia. Arch Bronconeumol. 2017;53:78–79. doi: 10.1016/j.arbres.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 13.McDermott S, Barry SC, Judge EP, et al. Tracheomalacia in adults with cystic fibrosis: Determination of prevalence and severity with dynamic cine CT. Radiology. 2009;252:577–86. doi: 10.1148/radiol.2522081956. [DOI] [PubMed] [Google Scholar]
- 14.Kandaswamy C, Balasubramanian V. Review of adult tracheomalacia and its relationship with chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2009;15:113–19. doi: 10.1097/MCP.0b013e328321832d. [DOI] [PubMed] [Google Scholar]
- 15.Lee KCH, Tan S, Goh JK, et al. Long-term outcomes of tracheobronchial stenosis due to tuberculosis (TSTB) in symptomatic patients: Airway intervention vs. conservative management. J Thorac Dis. 2020;12:3640–50. doi: 10.21037/JTD-20-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pathak V, Shepherd RW, Shojaee S. Tracheobronchial tuberculosis. J Thorac Dis. 2016;8:3818–25. doi: 10.21037/jtd.2016.12.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meghji J, Lesosky M, Joekes E, et al. Patient outcomes associated with post-tuberculosis lung damage in Malawi: A prospective cohort study. Thorax. 2020;75:269–78. doi: 10.1136/thoraxjnl-2019-213808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allwood B, van der Zalm M, Makanda G, et al. The long shadow post-tuberculosis. Lancet Infect Dis. 2019;19:1170–71. doi: 10.1016/S1473-3099(19)30564-X. [DOI] [PubMed] [Google Scholar]
- 19.Heimendinger E, Klotz G, Mounier-Kuhn P. [Tracheal stenosis and tracheomalacia in a fibrous tuberculosis patient]. Ann Otolaryngol. 1956;73:709–13. [in French] [PubMed] [Google Scholar]
- 20.Chung HS, Lee JH. Bronchoscopic assessment of the evolution of endobronchial tuberculosis. Chest. 2000;117:385–92. doi: 10.1378/chest.117.2.385. [DOI] [PubMed] [Google Scholar]
- 21.Low SY, Hsu A, Eng P. Interventional bronchoscopy for tuberculous tracheobronchial stenosis. Eur Respir J. 2004;24:345–47. doi: 10.1183/09031936.04.00003604. [DOI] [PubMed] [Google Scholar]
- 22.Su Z, Cheng Y, Wu Z, et al. Incidence and predictors of tracheobronchial tuberculosis in pulmonary tuberculosis: A multicentre, large-scale and prospective study in southern China. Respiration. 2019;97:153–59. doi: 10.1159/000492335. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Liu J, Chin DP. Progress in tuberculosis control and the evolving public-health system in China. Lancet. 2007;369:691–96. doi: 10.1016/S0140-6736(07)60316-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Zhang H, Ruan Y, et al. Tuberculosis prevalence in China, 1990–2010; A longitudinal analysis of national survey data. Lancet. 2014;383:2057–64. doi: 10.1016/S0140-6736(13)62639-2. [DOI] [PubMed] [Google Scholar]
- 25.Xin H, Zhang H, Liu J, et al. Mycobacterium tuberculosis infection among the elderly in 20 486 rural residents aged 50–70 years in Zhongmu county, China. Clin Microbiol Infect. 2019;25:1120–26. doi: 10.1016/j.cmi.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Pellegrini JM, Sabbione F, Morelli MP, et al. Neutrophil autophagy during human active tuberculosis is modulated by SLAMF1. Autophagy. 2020 doi: 10.1080/15548627.2020.1825273. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soehnlein O, Steffens S, Hidalgo A, et al. Neutrophils as protagonists and targets in chronic inflammation. Nat Rev Immunol. 2017;17:248–61. doi: 10.1038/nri.2017.10. [DOI] [PubMed] [Google Scholar]
- 28.Muefong CN, Sutherland JS. Neutrophils in tuberculosis-associated inflammation and lung pathology. Front Immunol. 2020;11:962. doi: 10.3389/fimmu.2020.00962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bickett TE, McLean J, Creissen E, et al. Characterizing the BCG induced macrophage and neutrophil mechanisms for defense against mycobacterium tuberculosis. Front Immunol. 2020;11:1202. doi: 10.3389/fimmu.2020.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thieblemont N, Wright HL, Edwards SW, et al. Human neutrophils in auto-immunity. Semin Immunol. 2016;28:159–73. doi: 10.1016/j.smim.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Xu Y, Zhang Q, Zhao Y. The functional diversity of neutrophils and clustered polarization of immunity. Cell Mol Immunol. 2020;17:1212–14. doi: 10.1038/s41423-020-0378-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demirin H, Ozhan H, Ucgun T, et al. Normal range of mean platelet volume in healthy subjects: Insight from a large epidemiologic study. Thromb Res. 2011;128:358–60. doi: 10.1016/j.thromres.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Zhong H, Zhao Y, et al. Role of platelet biomarkers in inflammatory response. Biomark Res. 2020;8:28. doi: 10.1186/s40364-020-00207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cognasse F, Laradi S, Berthelot P, et al. Platelet inflammatory response to stress. Front Immunol. 2019;10:1478. doi: 10.3389/fimmu.2019.01478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adatia K, Farag MF, Gue YX, et al. Relationship of platelet reactivity and inflammatory markers to recurrent adverse events in patients with ST-elevation myocardial infarction. Thromb Haemost. 2019;119:1785–94. doi: 10.1055/s-0039-1695007. [DOI] [PubMed] [Google Scholar]
- 36.Middleton EA, Weyrich AS, Zimmerman GA. Platelets in pulmonary immune responses and inflammatory lung diseases. Physiol Rev. 2016;96:1211–59. doi: 10.1152/physrev.00038.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrell CN, Pariser DN, Hilt ZT, et al. The platelet napoleon complex-small cells, but big immune regulatory functions. Annu Rev Immunol. 2019;37:125–44. doi: 10.1146/annurev-immunol-042718-041607. [DOI] [PubMed] [Google Scholar]
- 38.Büyükaşik Y, Soylu B, Soylu AR, et al. In vivo platelet and T-lymphocyte activities during pulmonary tuberculosis. Eur Respir J. 1998;12:1375–79. doi: 10.1183/09031936.98.12061375. [DOI] [PubMed] [Google Scholar]
- 39.Meyer A, Wang W, Qu J, et al. Platelet TGF-β1 contributions to plasma TGF-β1, cardiac fibrosis, and systolic dysfunction in a mouse model of pressure overload. Blood. 2012;119:1064–74. doi: 10.1182/blood-2011-09-377648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H, Hicks JJ, Wang, et al. Customized platelet-rich plasma with transforming growth factor β1 neutralization antibody to reduce fibrosis in skeletal muscle. Biomaterials. 2016;87:147–56. doi: 10.1016/j.biomaterials.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 41.Forcina L, Miano C, Scicchitano BM, et al. Signals from the Niche: Insights into the role of IGF-1 and IL-6 in modulating skeletal muscle fibrosis. Cells. 2019;8(3):232. doi: 10.3390/cells8030232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andrade D, Oliveira G, Menezes L, et al. Insulin-like growth factor-1 short-period therapy improves cardiomyopathy stimulating cardiac progenitor cells survival in obese mice. Nutr Metab Cardiovasc Dis. 2020;30:151–61. doi: 10.1016/j.numecd.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Squecco R, Chellini F, Idrizaj E, et al. Platelet-rich plasma modulates gap junction functionality and connexin 43 and 26 expression during TGF-β1-induced fibroblast to myofibroblast transition: clues for counteracting fibrosis. Cells. 2020;9:1199. doi: 10.3390/cells9051199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang T, Zhang J, Qiu XJ, et al. Scarring airway stenosis in Chinese adults: Characteristics and interventional bronchoscopy treatment. Chin Med J (Engl) 2018;131:276–81. doi: 10.4103/0366-6999.223850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khvilivitzky K, Trivedi PN, McFadden PM. Tuberculous tracheobronchial stenosis: Avoiding resection-when less is more. J Thorac Dis. 2017;9:E779–82. doi: 10.21037/jtd.2017.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]